Figure 2.
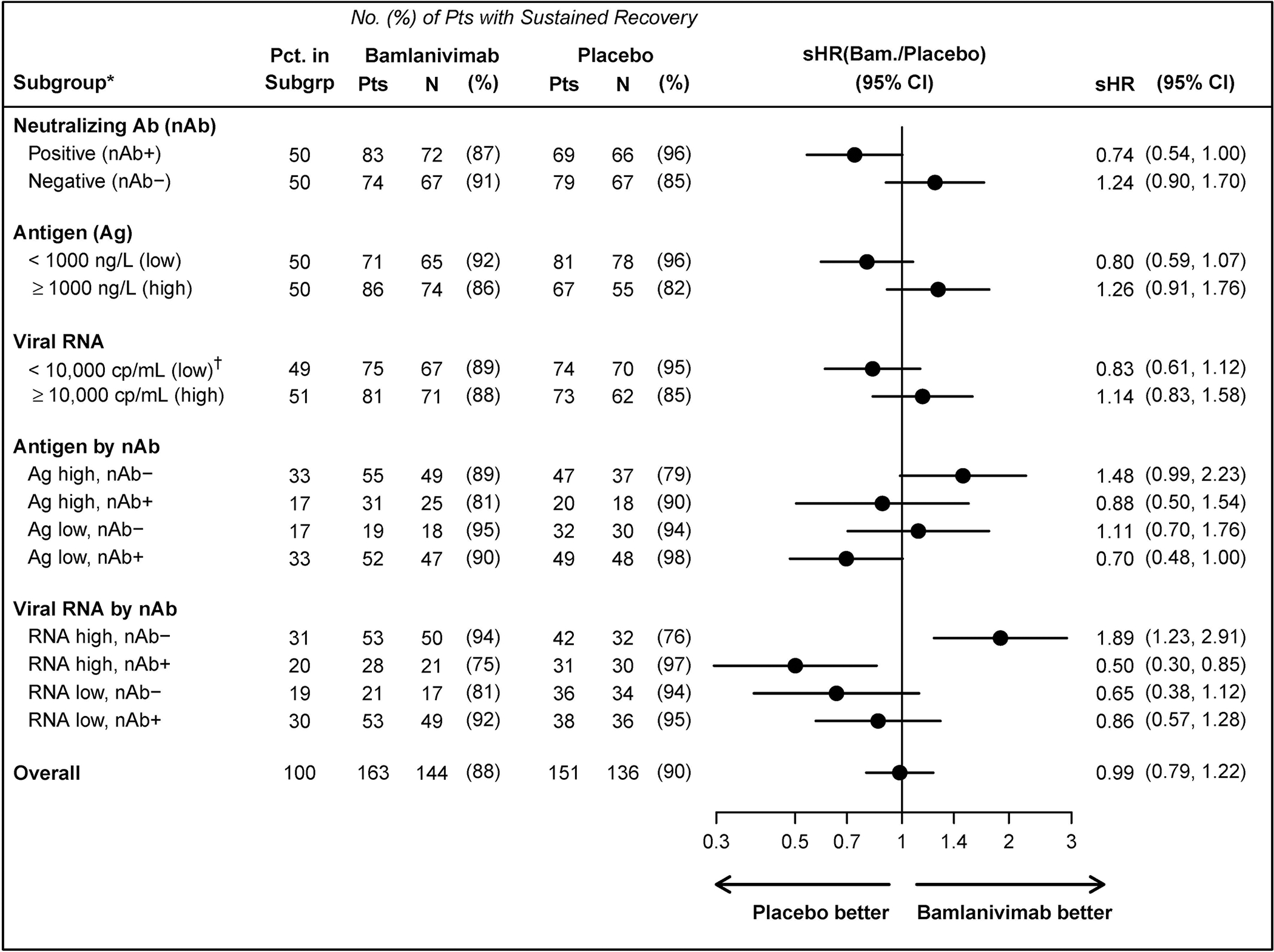
Sustained recovery according to subgroups at study entry: nAb status and levels of viral measures (plasma antigen and nasal viral RNA)+.
* Nominal P-values for differences in the treatment effect across subgroups (interactions between the subgroup indicator and treatment group indicator) are as follows: subgroups by nAb status, P=0.018; antigen level by nAb status, P=0.038; viral RNA level by nAb status, P<0.001.
† low viral RNA = viral RNA level < 10,000 cp/mL, negative, or indeterminate
+ See Appendix Figures S9–S11 for other subgroupings.
Abbreviations: Ag = plasma nucleocapside antigen levels at study entry; nAb = neutralising antibody status from a surrogate viral neutralization test at study entry; sHR=sub-hazard ratio for time to sustained recovery (also called “recovery rate ratio”); viral RNA = quantification of viral copies in fluid from nasal swab at study entry.
