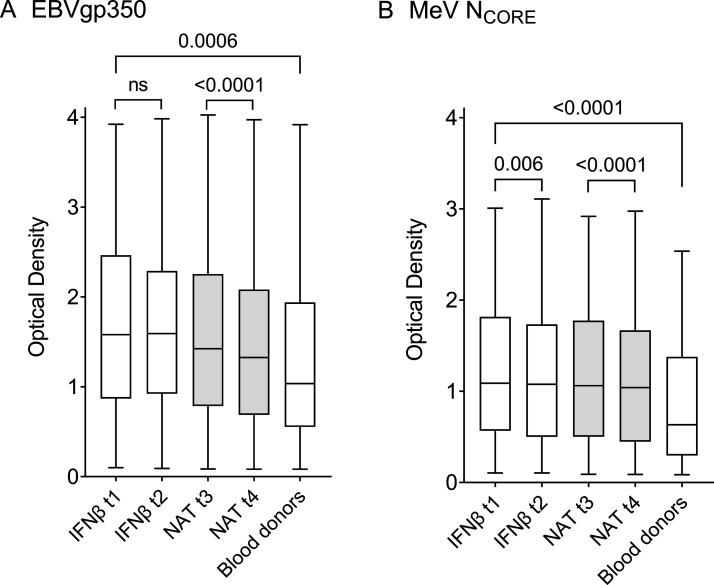
Figure 1.
IgG reactivity measured as optical density in serum samples against (A) Epstein-Barr virus glycoprotein 350 (EBVgp350) and (B) measles virus nucleocapsid protein (MeV NCORE). There were 170 patients with multiple sclerosis in the interferon beta (IFNβ) subgroup sampled at time point 1 (t1) and t2, 714 patients in the natalizumab (NAT) group, sampled at t3 and t4 and 144 blood donors. The boxplots demonstrate minimum, quartile 1, median, quartile 3 and maximum. The Mann-Whitney U test was used to compare the IgG levels in patients during IFNβ treatment at t1 and blood donors. The Wilcoxon signed-rank test was used to compare the anti-EBVgp350 and anti-MeV NCORE IgG levels between the samples collected during IFNβ treatment at t1 and t2 and before (t3) and during (t4) NAT therapy. P values<0.008 (0.05/6 due to Bonferroni correction) were considered statistically significant.