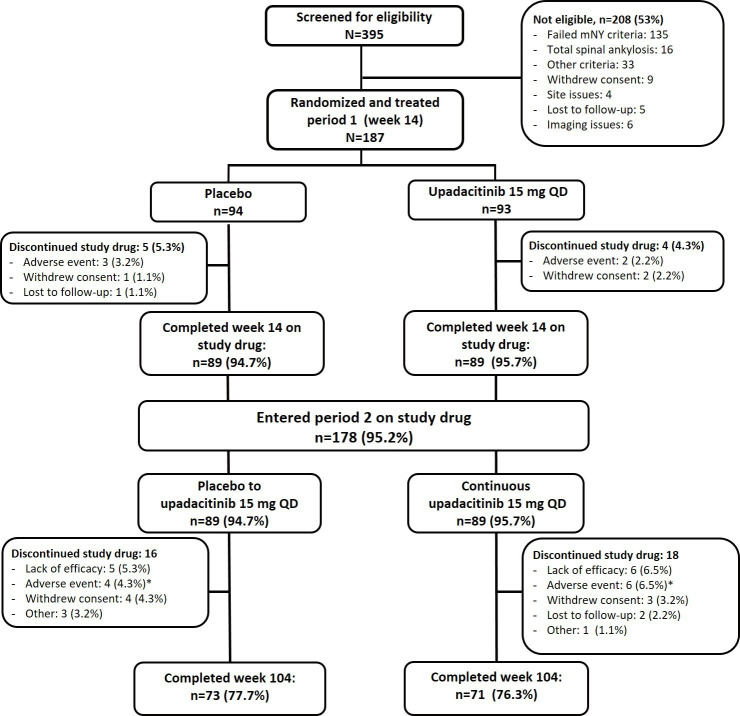
Figure 1.
Patient disposition through week 104. *AEs leading to discontinuation of study drug in period 2 in the continuous upadacitinib group were diarrhoea, headache, and vertigo (n=1), pulmonary embolism, herpes zoster, headache, squamous cell carcinoma of the tongue, and decreased haemoglobin (n=1 each) and in the placebo-to-upadacitinib group were hemiparesthesia and intervertebral disc protrusion(n=1), vasculitis, hyperplasia of prostate and anterior uveitis flare (n=1 each). mNY, modified New York; QD, once daily.