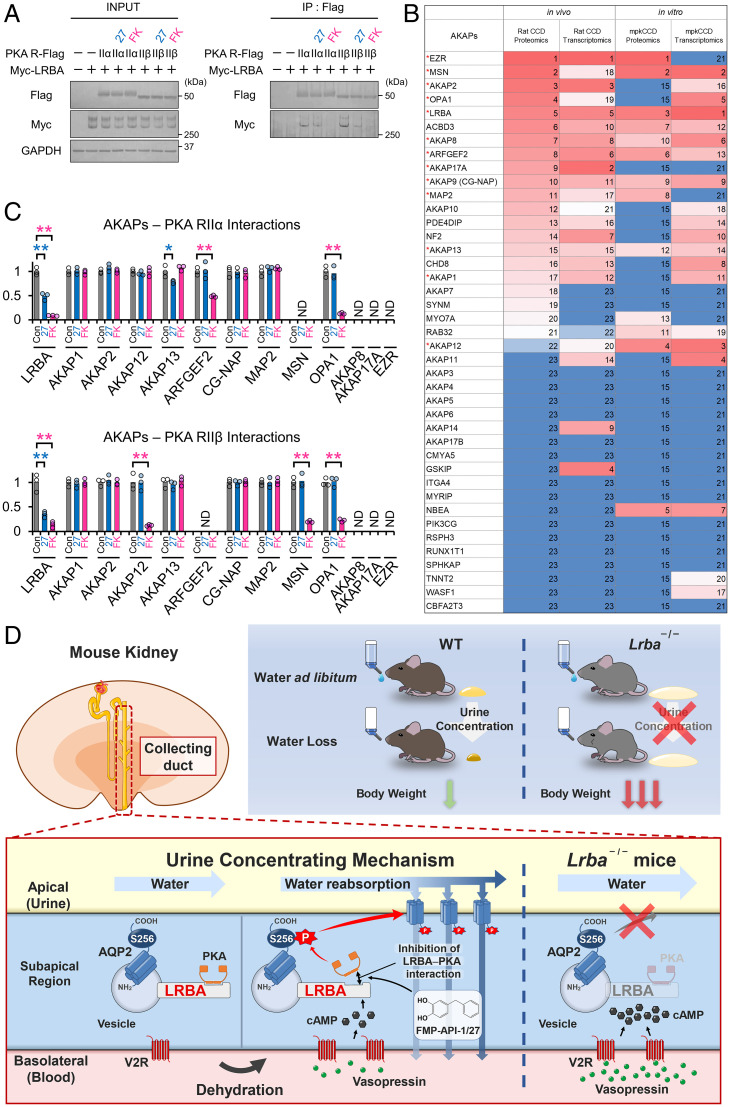
Fig. 6.
The LRBA–PKA interaction is the drug target of FMP-API-1/27. (A) FMP-API-1/27 dissociates the LRBA–PKA RIIβ interaction. PKA RII subunits (RIIα, RIIβ)-Flag and Myc-LRBA were overexpressed in HEK293T cells. FMP-API-1/27 (200 μM) or forskolin (10 μM) was added to the cells 1 h before coimmunoprecipitation. Representative blots of coimmunoprecipitated Myc-LRBA are shown (n = 3). (B) Highly expressed AKAP proteins and mRNA in renal collecting ducts are listed based on recent omics data (39–43). Expression levels are indicated by a color scale, with red representing relative high expression levels and blue indicating relative low expression levels. The numbers in the table indicate rank in sequential order. Highly expressed AKAPs, which are evaluated in C and SI Appendix, Fig. S10, are indicated by red asterisks. (C) FMP-API-1/27 and forskolin inhibit specific AKAP–PKA interactions. A coimmunoprecipitation assay for measuring AKAP–PKA interactions (SI Appendix, Fig. S10) was performed as in A. Densitometric analysis of coimmunoprecipitated AKAPs (n = 3). (D) Schematic summary of the physiological role of LRBA in the urine-concentrating system. Lrba−/− mice exhibited polyuria, and their urine-concentrating ability did not respond to vasopressin. As a result of diluted urine, serum vasopressin levels were elevated to compensate for water loss in Lrba−/− mice. Exclusion of PKA from the LRBA compartment by Lrba knockout caused a failure of PKA-induced AQP2 phosphorylation. The strength of LRBA–PKA interaction was dynamically changed by AQP2 activators. Vasopressin and FMP-API-1/27 dissociated PKA from LRBA, presumably to enhance AQP2 phosphorylation at S256. (C) Mean data are reported. **P < 0.05, *P < 0.01 by Tukey test. IP, immunoprecipitation; ND, AKAP–PKA interactions not detected.