Fig. 5.
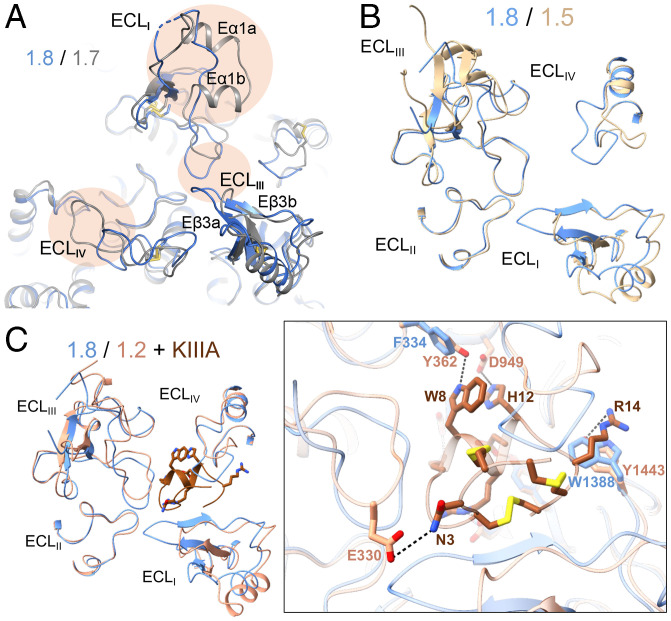
The ECLs above the PD may be exploited for the development of Nav1.8-specific suppressors. (A) The ECLs exhibit marked structural differences between Nav1.8 and Nav1.7. Shown here is a tilted extracellular view of the superimposed overall structures of class I Nav1.8 (blue) and Nav1.7 (gray) (PDB ID code: 7W9K). The structural distinctions of the ECL above the PD are highlighted with oval shades. (B) Nav1.8 and Nav1.5, both TTX-resistant subtypes, also deviate substantially in the ECL region. Shown here is an extracellular view of the ECLs from the superimposed overall structures of human Nav1.8 and Nav1.5 (PDB ID code: 6LQA). (C) The different structures of the ECLs underlie the distinct sensitivities of Nav1.8 and Nav1.2 to the peptide pore blocker µ-conotoxin KIIIA (KIIIA). (Inset) An extracellular view of the ECLs in the superimposed structures of Nav1.8 and KIIIA-bound Nav1.2 (PDB ID code: 6J8E). The elongated ECLI in Nav1.8 may interfere with KIIIA binding.