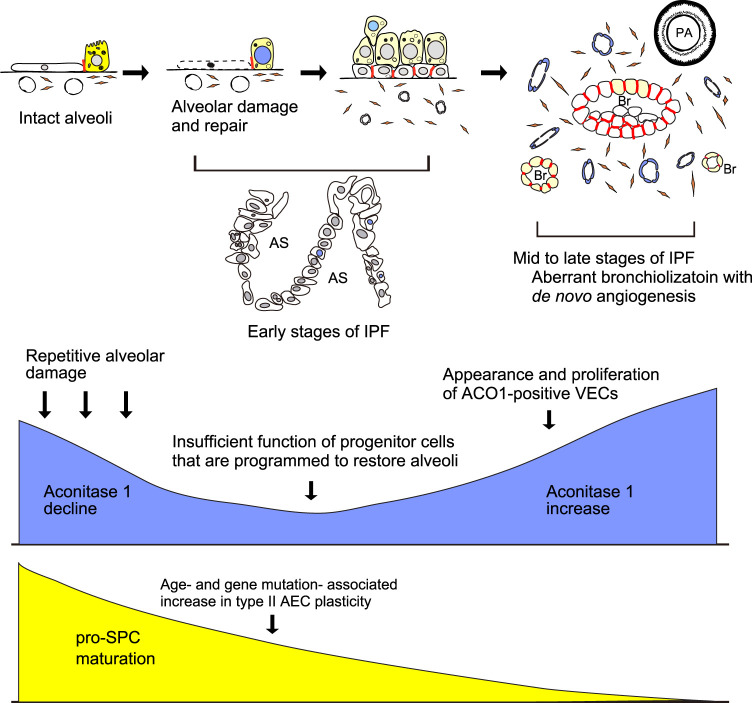
FIGURE 7.
Hypothesized mechanism of how aconitase 1 is involved in IPF development. Aging and genetic predispositions independently increase type II AEC plasticity. In parallel, repetitive injury to alveoli and the associated regeneration process gradually leads to the shortage of type II AEC-committed progenitor cells and accordingly loss of functionally mature type II AECs (early stages of IPF). Low expression of ACO1 in the upper lobes of IPF lungs (Figure 2E, F; Figure E1) corresponds to a paucity of ACO1-positive epithelial cells and the impaired transition from epithelial progenitor to functionally mature type II AECs. Once such disturbed type II AEC regeneration reaches a certain threshold, the conversion of alveoli into bronchioles starts to occur in a random manner. Such aberrant bronchiolization accompanies an uncontrolled de novo angiogenesis and fibrosis in the interstitium near the newly formed bronchioles (mid to late phases of IPF with high ACO1 expression). Yellow, blue, and red colors in the schema represent pro-SPC, ACO1, and E-cadherin respectively. AS, airspace; Br, Bronchiole; PA, pulmonary artery; AEC, alveolar epithelial cell; VECs, vascular endothelial cells.