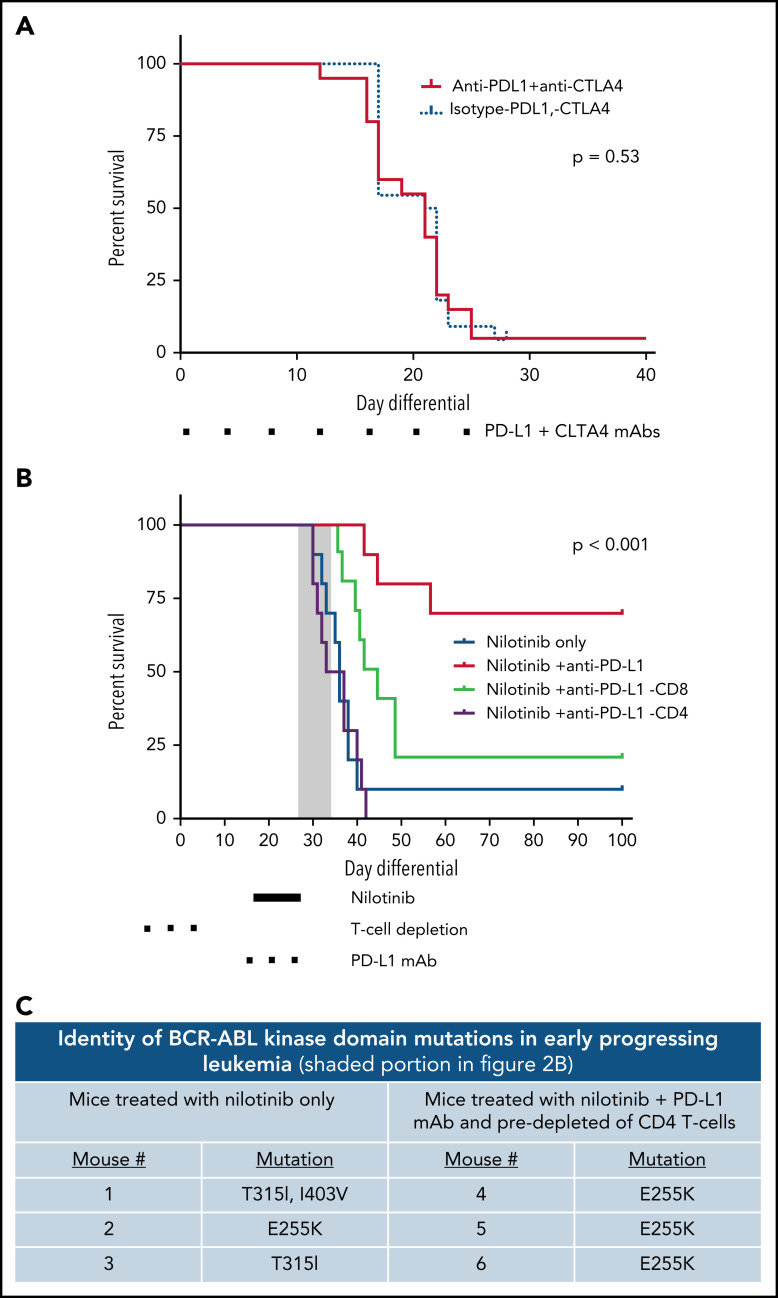
Figure 2.
Nilotinib plus PD-L1 blockade induces a protective anti-leukemia immune response in a CD4+ T-cell–dependent manner. (A) CD45.2+ mice were injected with 2500 LM138 leukemia cells. Dual PD-L1/CTLA-4 targeting antibody therapy (10 mg/kg each) was given simultaneously and continued twice per week. Statistical significance was analyzed using the Mantel-Cox Log-rank test. Results representative of 2 independent experiments (n = 10 mice per condition). (B) CD45.2+ mice were injected with 2500 LM138 leukemia cells. Starting at day 14 after leukemia injection, the mice were treated with either nilotinib and a PD-L1 blocking antibody (10 mg/kg) or nilotinib plus an isotype control antibody. In a cohort of mice, depleting antibodies against CD4+ or CD8+ T cells were administered before the start of treatment with nilotinib plus PD-L1 blockade. Survival of the mice in each of the treatment arms is shown. Statistical significance was analyzed using the Mantel-Cox log-rank test. Results representative of 2 independent experiments (n = 10 mice/arm). (C) Six mice became moribund while still receiving nilotinib or within 3 days of completing therapy (gray box indicated in 2B) and were euthanized. Spleens were harvested, genomic DNA was extracted, and the BCR-ABL kinase domain was amplified by polymerase chain reaction. Amplicons were cloned into plasmids that were transformed into Escherichia coli and streaked on agar plates. Individual colonies were picked, and plasmids sequenced in both forward and reverse directions using Sanger sequencing approaches. At least 2 clones per mouse were sequenced.