Abstract
The cytokine storm (CS) in hyperinflammation is characterized by high levels of cytokines, extreme activation of innate as well as adaptive immune cells and initiation of apoptosis. High levels of apoptotic cells overwhelm the proper recognition and removal system of these cells. Phosphatidylserine on the apoptotic cell surface, which normally provides a recognition signal for removal, becomes a target for hemostatic proteins and secretory phospholipase A2. The dysregulation of these normal pathways in hemostasis and the inflammasome result in a prothrombotic state, cellular death, and end-organ damage. In this review, we provide the argument that this imbalance in recognition and removal is a common denominator regardless of the inflammatory trigger. The complex reaction of the immune defense system in hyperinflammation leads to self-inflicted damage. This common endpoint may provide additional options to monitor the progression of the inflammatory syndrome, predict severity, and may add to possible treatment strategies.
Keywords: Inflammation, immunology, virology, apoptosis, phosphatidylserine exposure, secretory phospholipase A2
Impact Statement
The current pandemic of COVID-19 or SARS-CoV-2 infection has underscored the fact that the defense system that protects us can be overactivated which leads to an imbalance of protection and self-inflicted damage. The CS that invokes end-organ damage is not unique to COVID-19. Despite different triggers, a common denominator seems to correlate with clinical severity based on extreme apoptosis and ineffective removal of these apoptotic cells. Our defense system in hyperinflammation can be deemed appropriately exaggerated, but can also be pathologically devastating. Data are reviewed of examples of conditions that can lead to hyperinflammation and implicates possible ways to predict severity and may provide tools for the treatment of severely ill patients.
Introduction
Hyperactivation of innate and adaptive immune cells and highly elevated levels of circulating cytokines are often described under the umbrella term cytokine storm (CS) or cytokine release syndrome (CRS). 1 The hyperinflammatory syndrome presents an overlap between inflammatory responses that are deemed appropriately exaggerated and pathologically devastating. 2 The current COVID-19 pandemic has shown the complexity to clearly define the pathology and underlying mechanisms in which hyperinflammatory syndromes lead to end-organ damage via different immune mechanisms. 3 Bacterial or viral infections are well-recognized triggers of inflammation, but also abnormalities in immune response or human physiology can lead to devastating hyperinflammation.4,5 Apoptosis of stressed cells and proper removal is an important factor. While a proper activation of the apoptotic system is important, so is recognition and removal of apoptotic cells. Loss of normal phospholipid asymmetry in apoptosis provides an “eat me” signal to macrophages. While phosphatidylserine (PS) exposure is essential for platelets to induce the hemostatic process, unwanted PS exposure as the result of inadequate removal of apoptotic cells will result in a prothrombotic state, often correlated with severe inflammation-induced illness. Secretory phospholipase A2 (sPLA2), an essential secondary messenger in inflammation, 6 generates the building blocks for inflammatory signaling molecules like eicosanoids and platelet-activating factor (PAF). In addition, sPLA2 will hydrolyze bacterial and viral membranes, and as such assists in host defense. Unfortunately, sPLA2 will also effectively hydrolyze PS exposing (apoptotic) cells. This in turn will release uncontrolled cellular content and lipid breakdown products in the environment with the potential to stress and damage neighboring cells leading to a devastating downward spiral of cellular and organ damage.
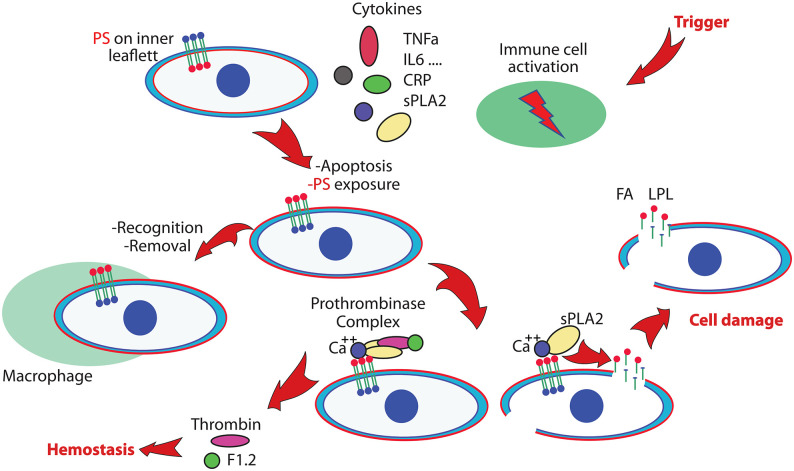
Figure 1 shows a working model to illustrate the link between the inflammasome, upregulated sPLA2 levels, induced apoptosis, cellular damage, and the prothrombotic state. The simplified presentation of these processes illustrates the normal reaction on an inflammatory trigger, and defines a common endpoint when the system reacts out of control. The immune system gears up to protect, immune cells respond, cytokines such as Tumor Necrosis Factor alpha (TNFα), Interleukin-6 (IL-6), and other messenger molecules, including C-reactive protein (CRP), is upregulated, sPLA2 provides building blocks for the generation of inflammatory compounds, and assists in the elimination of foreign entities like bacteria or viruses. In hyperinflammation, the normal and continuous process to remove unwanted apoptotic cells can get overwhelmed. The out of balance process to protect leads to a process that initiates a thrombotic state and lead to cellular damage and multiorgan failure.
Figure 1.
Simplified scheme of processes that will lead to vascular damage and multiorgan failure. An inflammatory trigger activates the immune system and initiates the production of several cytokines and the generation of secretory phospholipase A2 (sPLA2). Apoptotic processes activate phosphatidylserine (PS) exposure, a signal for cell removal. In hyperinflammation, overwhelming numbers of PS exposing cells that are not properly removed will imbalance the hemostatic system by unwanted activation of the prothrombinase complex. PS exposing cells are targets for sPLA2, leading to cell damage and generation of products including Fatty Acid (FA) and Lysophospholipid (LPL) that will damage additional cells. This can lead to a prothrombotic state and multiorgan failure. (A color version of this figure is available in the online journal.)
In this review, we attempt to make the case that despite the various triggers, variants in risk factors, and confusion regarding diagnosis of hyperinflammation pathology, the final step is common and centers on an out of balance system of apoptosis, PS exposure and significantly elevated sPLA2 levels in the inflammasome. This may point at a different way to monitor the clinical state of patients and suggests optional alternative approaches for treatment.
Plasma membrane phospholipids
The plasma membrane phospholipid distribution of animal cells is markedly asymmetric. The amino phospholipids PS and phosphatidylethanolamine (PE) are concentrated in the inner leaflet, whereas phosphatidylcholine (PC) and sphingomyelin (SM) are concentrated in the outer leaflet. Maintenance of membrane lipid asymmetry, and particularly the maintenance of PS in the inner leaflet, is a dynamic process that influences many events over the lifespan of the cell. Flippases or floppases are lipid pumps, that utilize ATP to translocate lipids between the bilayer leaflets. Scramblases, activated when physiologically required, transport lipids in both directions. Together with inhibition of the active inward movement, scrambling results in the loss of lipid asymmetry.7–9
The exposure of PS is an important physiologic event. On platelets, PS stimulates blood coagulation, and PS exposure by other cells during apoptosis provides an “eat me” signal to macrophages leading to “apoptotic clearance.” Programmed cell death is normally an orderly, energy-dependent process that causes cells that have completed their useful functions to die without inducing an inflammatory response. A diverse array of distinct and redundant receptor systems by phagocytes have developed, and provide an efficient recognition and elimination mechanism. The capacity of blood to form thrombin is a critical and a highly regulated determinant in the hemostatic process. PS exposure of platelets leads to plasma thrombin generation, as the prothrombinase complex binds in the presence of calcium to the negatively charged PS exposing membrane surface. The formation of this complex activates the cleavage of prothrombin to thrombin and fragment 1.2. Excessive and unwanted presence of PS exposure will lead to a prothrombotic state, 10 and lack of a proper removal of PS exposing cells can result in uncontrolled and persistent presentation of self-antigens to the immune system. 11
sPLA2 plays an important role in inflammation. The enzyme is a member of the large family of phospholipases that cleave the ester bond on the sn-2 position of phospholipids to generate lysophospholipid (LPL) and free fatty acid (FA). The different members of this family have different capabilities to attack cellular membranes. While the enzymes of snake and bee venom are potent in cellular attack of human membranes, the closely related pancreatic and secretory phospholipase isoforms will not randomly attack human tissue for obvious reasons. 12 However, sPLA2 will effectively hydrolyze bacterial and viral membranes and generate free arachidonic acid as building blocks for a large set of bioactive molecules. 6 sPLA2 levels are highly increased in acute clinical syndromes including sickle cell acute chest syndrome (ACS), pneumonia, acute asthma, and serious bacterial or viral infections.13–21 In contrast to normal cells, PS exposing membranes are rapidly broken down by sPLA2 in a calcium-dependent process. 22 This process can be inhibited by removal of calcium or the presence of proteins that bind in a calcium-mediated fashion to the PS exposing cell surface and “cloak” the PS exposing membrane from the sPLA2 attack or thrombotic activation. 23 Regardless of the trigger, elevated sPLA2 levels indicate a strong ongoing inflammatory signal and suggest a role of this enzyme in cell damage and organ failure.6,13,14,22,24,25 During convalescence, following acute illness, extreme levels of sPLA2 levels return to normal, but may remain elevated in chronic inflammatory conditions like rheumatoid arthritis 26 or sickle cell disease (SCD). 27 In contrast to the level of many cytokines that increases and decreases rapidly, the changes in time of the sPLA2 levels are more moderate. This makes measurement of sPLA2 levels in a good laboratory biomarker for the onset and timeline of clinical severity as was shown in the case of ACS in SCD.14,25,27
Endothelium plays a vital role in vascular physiological processes, but is also a major target to be considered in organ damage. Activation of endothelial cells leads to release of vasoactive substances including nitric oxide, PAF, prostacyclin, mitochondrial N-formyl peptide, and endothelin, as well as mediators of inflammation and thrombosis. Endothelial functions will be altered due to damage of the normal circulation. Ischemia of the endothelial cells and reperfusion due to resuscitation with fluids will induce endothelial cell apoptosis. Deprivation of oxygen required by endothelial cell mitochondria initiates an increase in mitochondrial reactive oxygen species and release of apoptogenic proteins. The structure of endothelium is compromised which, when not repaired, causes an impairment of the protective endothelial barrier resulting in increased permeability and leakage of fluids into the tissue.
Together the exposure of PS and proper recognition and removal of PS exposing apoptotic cells is essential. An imbalance in this highly orchestrated process can lead to a prothrombotic state, uncontrolled breakdown of cells, and generation of products that will lead to a devastating spiral of events that leads to end-organ damage as illustrated in Figure 1.
Hyperinflammation indicates a finely tuned defense system out of control, related to invasion of pathologic and/or deficiencies of the immune response. Sepsis and septic shock are the important causes of morbidity and lethality resulting from the extreme response to an infection. sPLA2 has been implicated as a mediator of organ failure associated with critical illness including intestinal disorders and life-threatening lung disorders such as acute lung injury (ALI) and the Acute Respiratory Distress Syndrome (ARDS).28,29 Since early identification of sepsis is critical, sPLA2 has been proposed as a predictor of sepsis and bacterial infection. 30
Hemophagocytic lymphohistiocytosis (HLH) is a highly fatal condition 31 as the result of mutations in immune cells leading to an abnormal response to inflammatory triggers.32,33 The unregulated activation of macrophages and T lymphocytes culminate in organ damage in part due to the inability to properly remove altered, apoptotic cells. The killing of target cells is a complex, multistage process that concludes with directed secretion of lytic granules-mediating apoptosis. Lack of lytic granules in natural killer (NK) cells is related to serious and often fatal diseases, such as familial hemophagocytic lymphohistiocytosis (FHL) or Griscelli syndrome type 2. 34 The X-linked inhibitor of apoptosis deficiency, XIAP (aka XLP-2), is a rare primary immunodeficiency frequently triggered by the Epstein–Barr virus (EBV) infection. XIAP is an antiapoptotic molecule, and recent findings demonstrate the role of XIAP in innate immunity and in the negative regulation of inflammation.35,36
SCD is marked by vaso-occlusive events as the result of a point mutation in globin which leads to hemoglobin polymerization, membrane changes, increased binding of blood cells to endothelium 37 resulting in an inflammatory and prothrombotic state, and organ damage related to ischemia reperfusion injury. 38 A specific case that illustrates the imbalance of the immune system is ACS in SCD which has parallels with the clinical presentation of ARDS, the devastating consequence of SARS-CoV-2 infection that has cost many lives in the last two years. Pulmonary fat embolism found in patients with ACS 39 results from vaso-occlusion and ischemia reperfusion of bone marrow, delivering an acute increase of apoptotic and necrotic material to the lung microcirculation. The inflammatory response and upregulated sPLA2 creates a situation where generation of LPL and FA increases in the circulation 27 and damages the endothelial and epithelium layer in the lung resulting in a rapidly developing clinical crisis. 40 The levels of sPLA2 and CRP have been shown to be harbingers of this serious condition.14,15,25,41 sPLA2 also correlates with postinjury multipole organ failure and is a marker of infection in febrile children presenting to a pediatric emergency department (ED).13,16
Viruses play a central role in devastating hyperinflammation in humans. Three examples are described in more detail that show the correlation with apoptosis, thrombosis, sPLA2, and end-organ damage. Viruses are opportunistic and unique intracellular pathogens as they fully rely on the host cell for their replication, to complete their lifecycle and potentiate disease. The continuous interactions between the human host and these pathogens during their coevolution have shaped both the immune system and the countermeasures used by pathogens. 42 Viruses hijack host cellular machinery for their replication and survival by targeting crucial cellular physiological pathways, including transcription, translation, immune pathways, and apoptosis. Aspects of three viruses with important public health impacts – Ebola, Dengue, and SARS-CoV-2 – are described. We argue that despite their obviously different modes of infection and pathology, they share a common final pathological feature. All can result in an extreme inflammatory response, unbridled formation of apoptotic cells, which in combination with high levels of sPLA2 and activation of hemostatic factors will play an important role in vascular pathology. Programmed cell death, a key component of the host innate immune response, is an effective strategy for the host cell to curb viral spread. However, many viruses are equipped to take advantage of these pathways in the host to subvert host defense and promote their own propagation. 43 In the past decade, emerging viral outbreaks like SARS-CoV-2, Zika, and Ebola have presented major challenges to the global health system and the current pandemic of COVID-19 has shown an intricate system that involves apoptosis, as well as the side effects of the antiviral strategy by the immune defense system. The choice to describe these three viruses is aimed to make the point of a common thread. Many other viral infections can be considered from a similar perspective. Influenza, commonly known as “the flu,” is a well-known vaccination target characterized by symptoms that range from mild to severe. Influenza infection is often mild and includes fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. However, it can also lead to serious complications in people who are at high risk. The raging Spanish flu pandemic that occurred a century ago can be considered as another example how we as humans succumb to our own out of balance defense system.
Ebola (EBOV) and Marburg (MARV) viruses are members of the Filoviridae family. EBOV, a single-stranded RNA virus, is highly pathogenic in humans. The virus induces excessive inflammation, lymphocyte apoptosis, hemorrhage, and coagulation defects, leading to multiorgan failure and shock. The acute infection results in death of more than 70% of patients.44–46 The most lethal strain has been associated with mortality rates of up to 91% in Zaire. 47 Most information on immune responses to EBOV comes from animal models and in vitro studies. Data on immune responses in humans is limited, due to biosafety requirements, logistics, and the fact that most outbreaks occur in remote areas. Studies in humans found that fatal outcome was associated with hypersecretion of numerous proinflammatory cytokines chemokines, growth factors as well as low levels of circulating cytokines produced by T lymphocytes. Fatal outcome is associated with aberrant innate immune responses and with global suppression of adaptive immunity. 48 Apoptosis is an important part of the host innate immune defense, especially against RNA viruses. Early target cells of filoviruses are monocytes, macrophages, and dendritic cells. EBOV proteins have been shown to induce apoptosis in recipient immune cells 49 and rapid depletion of T and NK lymphocytes by apoptosis. 50 Lymphocytes undergo massive and apparently unregulated apoptosis in human patients and laboratory animals, characterizing the terminal phase of fatal illness.51,52 Loss of lymphocytes in the host is thought to prevent the development of a functional adaptive immune response,53,54 and the degree of lymphopenia is highly correlative with fatalities. 55 The association of an early and sustained dysfunctional T-cell activation paralleled an overall CD4 T-cell decline. 56 Dendritic cells, which trigger the adaptive response, are infected and fail to mature appropriately, thereby impairing the T-cell response.57–59 Programmed death of human myeloid cells from different cell lines associated with ligand-induced TLR7/8-mediated inflammatory stress and depended on activation of apoptosis signal-regulating kinase 1 (ASK1). 60 Transcriptomics and protein quantification indicate that immature, proliferative monocyte-lineage cells with reduced antigen-presentation capacity replace conventional monocyte subsets, while lymphocytes upregulate apoptosis genes and decline in abundance. 61
The CS and immune cell apoptosis results in uncontrolled, systemic inflammation in affected individuals, resulting in apoptosis of cell populations different from immune. 62 Blocking lymphocyte apoptosis is not sufficient to improve survival in EBOV infection, 63 and fatal EBOV hemorrhagic fever results in massive intravascular apoptosis, which develops rapidly following infection and progresses relentlessly until death. 64 The initiation of a CS by the virus along with infection of endothelial cells leads to apoptosis and structural and functional changes that attenuate vascular integrity in many organs including the lungs, heart, liver, and kidney, and the damage of blood vessels of non-human primates at end-stage Ebola shows microvascular lesions and impaired blood supply to the organs further initiating damage. 65 Multiple hemorrhages were formed by diapedesis and fibrin deposition and thrombi were the features of hemostatic impairment, 66 as endothelial damage enhances the coagulation pathway leading to thrombus formation in major vessels and capillaries. 67 Apoptosis indicates that PS in the membrane of the host cell appears to play an important role in the life cycle of EBOV, 68 and lipidome changes were related to clinical outcome. 69 PE synergizes with PS to enhance PS receptor-mediated efferocytosis and virus entry.70,71 Plasma samples from patients infected with EBOV showed modified clotting times over time of infection, implicating PS exposing cells that trigger an unbalanced hemostasis. D-dimer levels elevated during infection with EBOV, returned to normal in patients who survived. 72 The use of thromboelastography showed that two infected patients had developed a marked hypercoagulable state early in their illness, which was treated with low-molecular-weight heparin. 73 Virtually no information is available on a potential role of sPLA2 in the vascular and organ damage observed. However, extreme upregulation of this enzyme can be expected and may confirm that the observed apoptosis, PS exposure, thrombotic events, and end-organ damage confirm the model as indicated in Figure 1.
Dengue virus (DENV) is one of the most prevalent mosquito-borne viral diseases of humans and a major public health issue with no effective vaccines in tropical and subtropical regions worldwide. 74 Aedes aegypti and Aedes albopictus, mosquitoes whose geographic range continues to expand, are the vectors for the four serotypes of the virus (DENV1 to DENV4). Humans are the only host for epidemic strains of DENV, and the continuous interactions between host and pathogens during their coevolution have shaped both the immune system and the countermeasures used by pathogens like DENV. 42 Host proteins are exploited by the virus in the course of infection as well as how the host counteracts the virus by eliciting different antiviral responses. 75 An estimated 2.5 billion people are at risk of dengue infection. Every year around 400 million people get infected by the DENV. Up to 500,000 develop dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS), the life-threatening forms of the infection with a mortality rate of about 20%.75,76 The large majority of DHF/DSS occurs as the result of a secondary infection with a different serotype of the virus. 77 Each serotype is antigenically different, meaning they elicit heterologous antibodies. Infection with one serotype will create neutralizing antibodies to the serotype. Cross-protection from other serotypes is not long term, instead heterotypic infection can cause severe disease. Mild disease is characterized by fever, pain behind the eyes, headache, muscle ache, joint pains, vomiting, and diarrhea. The progression to more severe forms of the disease associated with an extensive inflammatory response is still poorly understood. Prediction of severe dengue (DHF and DSS) in patients without clear warning signs has proven to be difficult.
The virus hijacks host cellular machinery for their replication and survival by targeting crucial cellular pathways,75,78 and the innate immune response to DENV infection induces transient immune aberrant activation, cytokine overproduction, and causes apoptosis and dysfunction of these cells. 79 DENV targets include dendritic reticulum cells, monocytes, lymphocytes, macrophages, hepatocytes, and vascular endothelial cells,80,81 and triggers multiple cell death pathways to produce several inflammatory cytokines that can lead to endothelial dysfunction, and therefore vascular leak. 82 Overproduced IL-6 might play a crucial role in activation of coagulation and fibrinolysis systems, and the enhanced production of sPLA2. DENV seems to efficiently adapt to and manipulate the host systems, and thus controls the survival of the infected cells.83–87 The PDZ domain-binding motif (PBM) in DENV proteins provides a high potential to influence the behavior of the host, based on any of the more than 400 cellular protein isoforms with a protein–protein interaction module (PDZ domain) in the human genome. 88 The DENV non-structural proteins manipulate interactions with several proteins to interfere with antiviral interferon signaling, 74 and inhibit the RNase Dicer. 89 DENV also utilizes various strategies to alter apoptotic pathways including autophagy temporarily sparing the infected cell, providing a suitable environment for the viral replication. 90 Virally induced apoptosis contributes directly to the cytopathogenic effects of DENV in cultured cells. Endothelium-related capillary leakage is triggered by DENV itself or by the host. DENV-induced vasculopathy and the unbalance between coagulation and fibrinolysis activation increase the likelihood of severe hemorrhage in DHF/DSS.77,82,91,92 Endothelial apoptosis may result in hypovolemic shock and downstream apoptosis of endothelium due to deprivation of oxygen required by endothelial cell mitochondria, setting the stage for a series of devastating events, damaging vascular viability in the pathophysiology in DHF and dengue with complication (DCC). 93 The DENV non-structural protein 1 (NS1), causes endothelial leakage, induces P-selectin expression and PS exposure in human platelets by interaction with TLR4, adenosine diphosphate (ADP)-induced platelet aggregation, and enhanced platelet adhesion to endothelial cells and phagocytosis by macrophages. 94 Limited data are available regarding the possible role of sPLA2 in dengue. Secreted phospholipases A2 from Viperid venoms with homology to human sPLA2 show ability to hydrolyze DENV suggesting that human sPLA2 could be involved in viral protection 95 by hydrolyzing the viral lipid bilayer. During the first 120 h of clinical illness, sPLA2 activity associated with PAF levels increases, suggesting a role for sPLA2 and PAF in vascular leak and the pathogenesis of dengue. 21 The coagulation and fibrinolysis systems are activated after DENV infection, and the unbalance between coagulation and fibrinolysis activation increases the likelihood of severe hemorrhage in DHF/DSS. Thrombocytopenia is a major characteristic observed in both mild and severe dengue disease and is significantly correlated with the progression of dengue severity. Together, the unbalanced formation of apoptotic cells, altered hemostasis upregulated sPLA2, and endothelial damage in severe dengue confirm to scheme of events in Figure 1.
COVID-19
A conclusive review to describe the molecular mechanisms that underlie the SARS-CoV-2-induced pandemic can only be incomplete at this time. It is hampered by the complexity of the enormous impact on public health, the arrival of new variants like the Delta variant in the second part of 2021 and the Omicron variant that is currently (January 2022) rapidly taking over as the main viral entity in COVID-19. From a biology perspective, we can only marvel at the effectiveness of this virus to mutate rapidly to a possible less severe but still devastating form, extremely effective in spreading through the human population in its own advantage. The rapid rate of new scientific findings and extreme volume of published new studies makes a conclusive detailed assessment difficult. However, the current studies clearly suggest a common denominator between COVID-19, Ebola and Dengue. Severe COVID is associated with hyperinflammation and a CS. 2 Disease severity and fatality is characterized by an association with thrombosis and vascular damage,3,96–100 the elevation of several cytokines (IP-10, MCP-3, and IL-1-ra CRP), and abnormal liver function. As is the case with the other intruders, COVID-19 intends to circumvent the human host defenses and the severe response leads to self-inflicted damage with devastating consequences that can be related to events depicted in Figure 1. The degree of viremia appears to relate to the clinical severity of COVID-19 and it is logical to assume that the number of apoptotic cells formed is also related to the viral dose. As is the case with Ebola or Dengue, viral infection leads to apoptosis, PS exposure, and is related to the macrophage removal of virus-infected cells.101–105 In a report focused on ACE2 receptors on endothelial cells in COVID-19, 106 apoptosis of endothelial cells and mononuclear cells was clearly apparent. Similarly, as observed for lung damage in SCD patients during ACS, endothelial damage and vascular dysfunction in COVID-19 will affect the vascular health in the lung as well as other organs including the brain. While information on the long-term COVID-19 problems is just emerging, it is tempting to assume that end-organ damage inflicted during the acute phase is the underlying reason for the observed clinical issues in COVID-19 survivors. As suggested under these conditions, when PS exposing cells are not efficiently removed, sPLA2 will break down cells that expose PS. 22 Lipolysis of these damaged cells by sPLA2 will generate non-esterified fatty acids (NEFA) and LPLs and lead to release of cellular content in the environment. These sPLA2-induced cellular breakdown products will affect other cells in the circulation when not properly buffered or removed. A clear correlation was reported between levels of CRP and sPLA2 in patients with SCD that develop ACS. 41 CRP provides a binding site for LPL, and recent data have shown a possible correlation between levels of CRP and severity in subsets of COVID-19 patients.107–109 The correlation between upregulated CRP and severity is, however, not always clear. An extensive formation of LPL and FA will overwhelm the normal buffering of sPLA2 products by CRP and albumin or lipoproteins. sPLA2 levels are correlated to clinical severity in the COVID-19 population, including a pediatric subset.17,19,110–114 Even though they represent only a fraction of the total severe COVID-19 cases, pediatric COVID-19 has become a major public health issue. Diorio et al. 115 demonstrated an elevation in cytokine profiles associated with multisystem inflammatory syndrome in children (MIS-C), in children infected with COVID-19, suggesting that early identification of inflammatory factors like sPLA2 is valuable to monitor disease severity. Recent studies have found a considerable number of hospitalized and critically ill pediatric patients.116–118 This seems even more the case with the current omicron variant. Underlying medical conditions, including immune compromise and cardiorespiratory comorbidities, appear to be at increased risk of pediatric COVID-19. 119 Nevertheless, the association of MIS-C with COVID-19 seems also related to previously healthy children with no underlying comorbidities. 120 MIS-C, a postinfectious hyperinflammatory response to SARS-CoV-2 infection, 121 shows high rates of pediatric intensive care unit (PICU) admission, vasopressor requirements, and mechanical ventilation,122,123 and includes myocarditis, cardiorespiratory failure, and even death. 124 A biomarker of pediatric COVID-19 and MIS-C disease severity to guide patient management would be highly valuable to stratify risk. New evidence is emerging daily that COVID-19 is more than a respiratory disease; it can result in coagulopathy, multiorgan failure and in severely ill patients, and circulatory damage may underlie the still poorly understood long COVID syndrome.
Taken together, the examples of viral-induced inflammation suggest that despite very different molecular and clinical processes, the end result of hyperinflammation appears the inability to remove PS exposing (apoptotic) cells resulting in a prothrombotic state which in combination with sPLA2 can lead to cellular damage, endothelial dysfunction, and end-organ damage
Severity prediction
A clear link between biomarkers and prediction of clinical severity as a diagnostic tool in hyperinflammation has been difficult. A large variety of cytokines and other markers like CRP in addition to white blood cell assessment have been used as biomarkers in inflammation but often fail to link to outcome. Many of the cytokines have short response times. They increase and decrease rapidly making timed sampling complex. sPLA2 has a much slower response and measuring its levels in blood has been used to predict the onset of ACS in SCD. Assessment of apoptosis in vivo has been difficult. PS exposure to identify apoptotic cells in vitro can be readily assessed by the use of fluorescently labeled Annexin V. As a fluorescent derivative annexin is a widely used to visualize apoptotic cells by microscopy or flowcytometry. 125 Radiolabeled Annexin V can be used for in vivo studies in animals. Assessment of PS exposure in blood cells can be accomplished ex vivo, 126 but direct assessment of, for example, endothelium PS exposure in patients is not possible. Indirect assessment of PS exposure can be pursued. Thrombin enters the coagulation cascade after binding of the prothrombinase complex to a PS exposing surface and release of Fragment 1.2 from prothrombin. Downstream biomarkers of thrombotic events like Di-dimer are routinely used as biomarkers, but a more direct approach by measuring F1.2 as the initial step of PS exposure could be contemplated. Similarly, if one assumes that sPLA2-induced damage is a major factor in cellular damage, it seems logical to track the levels of sPLA2 in patients. In SCD, for instance, daily assessment of sPLA2 levels predicted the onset of ACS.14,15,25,27 The assay to measure sPLA2 can easily be introduced as a routine assay in the acute care setting, added to the measurements provided by a clinical lab. The sPLA2 results may avoid delayed diagnosis of hyperinflammatory-related pathology as observed in COVID-19 127 and could provide a tool for the clinician to decide on a course of action to benefit the patient.
Treatment
While viremia obviously triggers the pathology observed in viral infections, clinical data suggest that the response by the immune system plays an important role in the morbidity and mortality of COVID-19. This in turn has generated interest in treatments that mitigate the immune response including corticosteroids and other immunomodulatory agents. However, treatments that act broadly to suppress the immune system have the potential to impede the body’s ability to control the viral infection, and there is no clear consensus as to which inflammatory biomarkers identify appropriate anticytokine treatment. Severely ill COVID-19 patients have been treated with general anti-inflammatory drugs that target various cytokines. However, since cytokines are also essential in eliminating SARS-COV-2, blocking these cytokines pathways could potentially lead to unwanted and poor outcomes. Based on measured CRP levels, 128 anti-inflammatory corticosteroids have only led to improved survival in a subset of patients. Drugs that target specific cytokines or cytokine pathways have been only partly successful. Anakinra which blocks both IL-1α and IL-1β seemed to improve survival.129,130 However, a double-blind study of canakinumab, a monoclonal antibody to IL-1β&0x44; failed to show a improved survival. 131 Whereas Janus kinase inhibition which targets multiple inflammatory cytokines 132 seemed to improve survival, 133 antibodies to either IL-6 or its receptor did not show a clear benefit. 128 Since its discovery in the serum of patients with severe inflammation and in rheumatoid arthritic fluids, sPLA2 as a proinflammatory enzyme has invoked intense interest in selective inhibitors of sPLA2-IIA in the hope of developing new and efficient therapies for inflammatory diseases. 134 Corticosteroids reduce the formation of sPLA2 and thereby may benefit severe cases of COVID-19,135–139 but their use has also been related to adverse effects, 140 as shown in a large cohort of relatively healthy patients from Taiwan. 140 Treatments that address specific inflammatory pathways may be more advantageous.
Since blood levels of sPLA2 correlate with severity, addressing measures to lower elevated levels of sPLA2, together with apoptotic changes in cellular membranes, appears logical. Compounds that affect the formation of sPLA2 including IL-6 blockers such as sirukumab, MEDI5117, sarilumab, ALX-0061, clazakizumab, and olokizumab 141 as well as IL-6 inhibitors such as tocilizumab (TCZ) could be considered. Treatment of a number of autoimmune diseases including ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, psoriasis, psoriatic arthritis, and Behçet’s disease have included the use of infliximab, a chimeric monoclonal TNF-alpha antibody, by action that has been related to a decrease in the formation of sPLA2. 142 Compounds like Varespladib, an sPLA2 inhibitor, 143 and other specific inhibitors of sPLA2 may warrant further investigation as therapeutic agents.
In 2021, a clinical study for the use of Varespladib in COVID-19 was announced (https://clinicaltrials.gov/ct2/show/NCT04969991). The use of an sPLA2 inhibitor in sepsis demonstrated improved survival in a subgroup of patients who received the drug within 24 h of sepsis-induced organ failure. Results in a larger group with severe organ failure was not significant, 144 confirming that the administration of these compounds is needed before major organ damage has occurred. Diagnostic assessment of sPLA2 levels together with the use of sPLA2 inhibitors may allow a better clinical management.
PS exposure of cells triggers both the sPLA2-induced cellular damage and the prothrombotic state. Therefore, “cloaking” of PS surfaces may represent a strategy to mitigate both dysregulation of hemostasis and sPLA2 attack. Careful management is obviously required. Whereas PS exposure on cells other than platelets may lead to a prothrombotic state, the assembly of the prothrombinase complex on the PS exposing surface of activated platelets is essential for normal hemostasis. The current findings in the COVID-19 pandemic seem to confirm the relationship of apoptosis and hemostatic disorder. Abnormal coagulation parameters in COVID-19 patients from Wuhan 99 were confirmed with additional studies.145,146 Both thrombus formation and elevation of D-dimer was reported in COVID-19. 147 Treatment with low molecular weight heparin appeared to associate with outcomes. 97 Annexin is a common name for a group of cellular proteins that bind to PS exposing membranes in the presence of calcium, and Di-annexin was generated to provide a stronger binding and longer lifetime in the circulation to “cloak” PS exposing surfaces. 23 This compound has proven to be effective in modulation ischemia reperfusion injury in animals,148–153 and has been used in solid organ transplants.150–155 We speculate that this compound could lower both the damage invoked by sPLA2 as well as modulate the onset of thrombotic events.
Ultimately, treatment options for several of these devastating inflammatory conditions will rely on a combination of modalities to avoid the onset of hyperinflammation. This obviously includes measures to avoid infection such as vaccines and other public health measures. Lowering the risk of hyperinflammation includes treatments that inhibit, for example, viral replication, and anticytokine and anti-inflammatory agents. However, for the critically sick patients, the tools in the hands of physicians are limited. One can argue that compounds that specifically target sPLA2 and PS exposing cells may benefit these patients and may also have a positive effect on long-term damage as is observed in patients after the resolve of the acute illness.
In conclusion, measurement of sPLA2 and factors that report on the onset of vascular damage in combination with specific therapeutic compounds targeting sPLA2, as well as those that can modulate vascular damage as the result of the presence of PS exposing cells, warrant consideration to modulate the devastation effects of hyperinflammation.
Footnotes
Authors’ Contributions: F.A.K. wrote and edited the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Frans A Kuypers  https://orcid.org/0000-0003-0731-1007
https://orcid.org/0000-0003-0731-1007
References
- 1. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect 2020;80:607–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol 2020;20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ponnatt TS, Lilley CM, Mirza KM. Hemophagocytic lymphohistiocytosis. Arch Pathol Lab Med 2021;146:507–19 [DOI] [PubMed] [Google Scholar]
- 5. Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 2012;63:233–46 [DOI] [PubMed] [Google Scholar]
- 6. Glaser KB, Vadas P. Phospholipase A2 in clinical inflammation: molecular approaches to pathophysiology (Pharmacology and toxicology). Boca Raton, FL: CRC Press, 1995, pp. xiv, 205 [Google Scholar]
- 7. Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta 1999;1439:317–30 [DOI] [PubMed] [Google Scholar]
- 8. Clarke RJ, Hossain KR, Cao K. Physiological roles of transverse lipid asymmetry of animal membranes. Biochim Biophys Acta Biomembr 2020;1862:183382. [DOI] [PubMed] [Google Scholar]
- 9. Shin HW, Takatsu H. Phosphatidylserine exposure in living cells. Crit Rev Biochem Mol Biol 2020;55:166–78 [DOI] [PubMed] [Google Scholar]
- 10. Wan J, Konings J, de Laat B, Hackeng TM, Roest M. Added value of blood cells in thrombin generation testing. Thromb Haemost 2021;121: 1574–87 [DOI] [PubMed] [Google Scholar]
- 11. Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: a matter of life and death. Annu Rev Physiol 2003;65:701–34 [DOI] [PubMed] [Google Scholar]
- 12. Villarrubia VG, Costa LA, Diez RA. [Secreted phospholipases A2 (sPLA2): friends or foes? Are they actors in antibacterial and anti-HIV resistance?]. Med Clin (Barc) 2004;123:749–57 [DOI] [PubMed] [Google Scholar]
- 13. Partrick DA, Moore EE, Silliman CC, Barnett CC, Kuypers FA. Secretory phospholipase A2 activity correlates with postinjury multiple organ failure. Crit Care Med 2001;29:989–93 [DOI] [PubMed] [Google Scholar]
- 14. Ballas SK, Files B, Luchtman-Jones L, Benjamin L, Swerdlow P, Hilliard L, Coates T, Abboud M, Wojtowicz-Praga S, Kuypers FA, Michael Grindel J. Secretory phospholipase A2 levels in patients with sickle cell disease and acute chest syndrome. Hemoglobin 2006;30:165–70 [DOI] [PubMed] [Google Scholar]
- 15. Styles LA, Abboud M, Larkin S, Lo M, Kuypers FA. Transfusion prevents acute chest syndrome predicted by elevated secretory phospholipase A2. Br J Haematol 2007;136:343–4 [DOI] [PubMed] [Google Scholar]
- 16. Mansour KM, Kuypers FA, Wang TN, Miller AM, Larkin SK, Morris CR. Secretory phospholipase A2: a marker of infection in febrile children presenting to a pediatric ED. Am J Emerg Med 2011;29:1163–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snider JM, You JK, Wang X, Snider AJ, Hallmark B, Zec MM, Seeds MC, Sergeant S, Johnstone L, Wang Q, Sprissler R, Carr TF, Lutrick K, Parthasarathy S, Bime C, Zhang HH, Luberto C, Kew RR, Hannun YA, Guerra S, McCall CE, Yao G, Del Poeta M, Chilton FH. Group IIA secreted phospholipase A2 is associated with the pathobiology leading to COVID-19 mortality. J Clin Invest 2021;131:e149236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimura T, Kurano M, Okamoto K, Jubishi D, Kano K, Igarashi K, Shimamoto S, Aoki J, Moriya K, Yatomi Y. Increase in serum levels of phosphatidylserine-specific phospholipase A1 in COVID-19 patients. Cell Mol Immunol 2021;18:2275–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuypers FA, Rostad CA, Anderson EJ, Chahroudi A, Jaggi P, Wrammert J, Mantus G, Basu R, Harris F, Hanberry B, Camacho-Gonzalez A, Manoranjithan S, Vos M, Brown LA, Morris CR. Secretory phospholipase A2 in SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-C). Exp Biol Med (Maywood) 2021;246:2543–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malavige GN, Ogg GS. Pathogenesis of vascular leak in dengue virus infection. Immunology 2017;151:261–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeewandara C, Gomes L, Udari S, Paranavitane SA, Shyamali NL, Ogg GS, Malavige GN. Secretory phospholipase A2 in the pathogenesis of acute dengue infection. Immun Inflamm Dis 2017;5:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neidlinger NA, Larkin SK, Bhagat A, Victorino GP, Kuypers FA. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J Biol Chem 2006;281:775–81 [DOI] [PubMed] [Google Scholar]
- 23. Kuypers FA, Larkin SK, Emeis JJ, Allison AC. Interaction of an annexin V homodimer (Diannexin) with phosphatidylserine on cell surfaces and consequent antithrombotic activity. Thromb Haemost 2007;97:478–86 [PubMed] [Google Scholar]
- 24. Li W, Zhang M, Guo Y, Liu X, Ji X, Su J, Zhang Z, Zhang F. Serum secretory phospholipase A2 group IB correlates with the severity of membranous nephropathy. Clin Chim Acta 2018;482:178–84 [DOI] [PubMed] [Google Scholar]
- 25. Styles LA, Aarsman AJ, Vichinsky EP, Kuypers FA. Secretory phospholipase A(2) predicts impending acute chest syndrome in sickle cell disease. Blood 2000;96:3276–8 [PubMed] [Google Scholar]
- 26. Duchez AC, Boudreau LH, Naika GS, Rousseau M, Cloutier N, Levesque T, Gelb MH, Boilard E. Respective contribution of cytosolic phospholipase A2alpha and secreted phospholipase A2 IIA to inflammation and eicosanoid production in arthritis. Prostaglandins Other Lipid Mediat 2019;143:106340. [DOI] [PubMed] [Google Scholar]
- 27. Kuypers FA, Styles L, Schalkwijk CG, Hassel KL, Vichinsky E, Lubin BH, Weil JV, Lane PA. Increased serum levels of phospholipase A2 and free fatty acid in sickle cell disease associated with acute chest syndrome (ACS). In: Beuzard Y, Lubin BH, Rosa J. (eds) Sickle cell disease and thalassaemias: new trends in therapy. Paris: Colloque INSERM/John Libbey Eurotext Ltd., 1995, pp.501–3 [Google Scholar]
- 28. Letsiou E, Htwe YM, Dudek SM. Secretory phospholipase A2 enzymes in acute lung injury. Cell Biochem Biophys 2021;79:609–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corke C, Glenister K, Watson T. Circulating secretory phospholipase A2 in critical illness – the importance of the intestine. Crit Care Resusc 2001;3:244–9 [PubMed] [Google Scholar]
- 30. Berg E, Paukovits J, Axelband J, Trager J, Ryan D, Cichonski K, Kopnitsky M, Zweitzig D, Jeanmonod R. Measurement of a novel biomarker, secretory phospholipase A2 group IIA as a marker of sepsis: a pilot study. J Emerg Trauma Shock 2018;11:135–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah AR, Muzzafar T, Assi R, Schellingerhout D, Estrov Z, Tamamyan G, Kantarjian H, Daver N. Hemophagocytic lymphohistiocytosis in adults: an under recognized entity. BBA Clin 2017;7:36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. La Rosee P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019;133:2465–77 [DOI] [PubMed] [Google Scholar]
- 33. Carvelli J, Piperoglou C, Farnarier C, Vely F, Mazodier K, Audonnet S, Nitschke P, Bole-Feysot C, Boucekine M, Cambon A, Hamidou M, Harle JR, de Saint Basile G, Kaplanski G. Functional and genetic testing in adults with HLH reveals an inflammatory profile rather than a cytotoxicity defect. Blood 2020;136:542–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Front Immunol 2012;3:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Latour S, Aguilar C. XIAP deficiency syndrome in humans. Semin Cell Dev Biol 2015;39:115–23 [DOI] [PubMed] [Google Scholar]
- 36. Lekbua A, Ouahed J, O’Connell AE, Kahn SA, Goldsmith JD, Imamura T, Duncan CN, Kelsen JR, Worthey E, Snapper SB, Softic S. Risk-factors associated with poor outcomes in VEO-IBD secondary to XIAP deficiency: a case report and literature review. J Pediatr Gastroenterol Nutr 2019;69:e13–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuypers FA. Hemoglobin s polymerization and red cell membrane changes. Hematol Oncol Clin North Am 2014;28:155–79 [DOI] [PubMed] [Google Scholar]
- 38. Vichinsky E. Emerging herapies targetting the pathophysiology of sickle cell disease. Hematology/oncology clinics of North America. Amsterdam: Elsevier, 2014 [Google Scholar]
- 39. Tsitsikas DA, Vize J, Abukar J. Fat embolism syndrome in sickle cell disease. J Clin Med 2020;9:3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vichinsky E, Williams R, Das M, Earles AN, Lewis N, Adler A, McQuitty J. Pulmonary fat embolism: a distinct cause of severe acute chest syndrome in sickle cell anemia. Blood 1994;83:3107–12 [PubMed] [Google Scholar]
- 41. Bargoma EM, Mitsuyoshi JK, Larkin SK, Styles LA, Kuypers FA, Test ST. Serum C-reactive protein parallels secretory phospholipase A2 in sickle cell disease patients with vasoocclusive crisis or acute chest syndrome. Blood 2005;105:3384–5 [DOI] [PubMed] [Google Scholar]
- 42. Mancini M, Vidal SM. Mechanisms of natural killer cell evasion through viral adaptation. Annu Rev Immunol 2020;38:511–39 [DOI] [PubMed] [Google Scholar]
- 43. Nguyen LN, Kanneganti TD. PANoptosis in viral infection: the missing puzzle piece in the cell death field. J Mol Biol 2022;434:167249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menicucci AR, Jankeel A, Feldmann H, Marzi A, Messaoudi I. Antiviral innate responses induced by VSV-EBOV vaccination contribute to rapid protection. mBio 2019;10:e00597–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med 1999;5:423–6 [DOI] [PubMed] [Google Scholar]
- 46. Bray M, Geisbert TW. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol 2005;37:1560–6 [DOI] [PubMed] [Google Scholar]
- 47. Sheng M, Zhong Y, Chen Y, Du J, Ju X, Zhao C, Zhang G, Zhang L, Liu K, Yang N, Xie P, Li D, Zhang MQ, Jiang C. Hsa-miR-1246, hsa-miR-320a and hsa-miR-196b-5p inhibitors can reduce the cytotoxicity of Ebola virus glycoprotein in vitro. Sci China Life Sci 2014;57:959–72 [DOI] [PubMed] [Google Scholar]
- 48. Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal Zaire Ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis 2010;4:e837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pleet ML, DeMarino C, Stonier SW, Dye JM, Jacobson S, Aman MJ, Kashanchi F. Extracellular vesicles and Ebola virus: a new mechanism of immune evasion. Viruses 2019;11:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bradfute SB, Braun DR, Shamblin JD, Geisbert JB, Paragas J, Garrison A, Hensley LE, Geisbert TW. Lymphocyte death in a mouse model of Ebola virus infection. J Infect Dis 2007;196 Suppl 2:S296–304 [DOI] [PubMed] [Google Scholar]
- 51. Menicucci AR, Versteeg K, Woolsey C, Mire CE, Geisbert JB, Cross RW, Agans KN, Jankeel A, Geisbert TW, Messaoudi I. Transcriptome analysis of circulating immune cell subsets highlight the role of monocytes in Zaire Ebola Virus Makona pathogenesis. Front Immunol 2017;8:1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parrino J, Hotchkiss RS, Bray M. Prevention of immune cell apoptosis as potential therapeutic strategy for severe infections. Emerg Infect Dis 2007;13:191–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gupta M, Spiropoulou C, Rollin PE. Ebola virus infection of human PBMCs causes massive death of macrophages, CD4 and CD8 T cell sub-populations in vitro. Virology 2007;364:45–54 [DOI] [PubMed] [Google Scholar]
- 54. Bradfute SB, Warfield KL, Bavari S. Functional CD8+ T cell responses in lethal Ebola virus infection. J Immunol 2008;180:4058–66 [DOI] [PubMed] [Google Scholar]
- 55. Iampietro M, Younan P, Nishida A, Dutta M, Lubaki NM, Santos RI, Koup RA, Katze MG, Bukreyev A. Ebola virus glycoprotein directly triggers T lymphocyte death despite of the lack of infection. PLoS Pathog 2017;13:e1006397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Agrati C, Castilletti C, Casetti R, Sacchi A, Falasca L, Turchi F, Tumino N, Bordoni V, Cimini E, Viola D, Lalle E, Bordi L, Lanini S, Martini F, Nicastri E, Petrosillo N, Puro V, Piacentini M, Di Caro A, Kobinger GP, Zumla A, Ippolito G, Capobianchi MR. Longitudinal characterization of dysfunctional T cell-activation during human acute Ebola infection. Cell Death Dis 2016;7:e2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ilinykh PA, Lubaki NM, Widen SG, Renn LA, Theisen TC, Rabin RL, Wood TG, Bukreyev A. Different temporal effects of Ebola virus VP35 and VP24 proteins on global gene expression in human dendritic cells. J Virol 2015;89:7567–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lubaki NM, Ilinykh P, Pietzsch C, Tigabu B, Freiberg AN, Koup RA, Bukreyev A. The lack of maturation of Ebola virus-infected dendritic cells results from the cooperative effect of at least two viral domains. J Virol 2013;87:7471–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lubaki NM, Younan P, Santos RI, Meyer M, Iampietro M, Koup RA, Bukreyev A. The Ebola interferon inhibiting domains attenuate and dysregulate cell-mediated immune responses. PLoS Pathog 2016;12:e1006031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nicholas SA, Oniku AE, Sumbayev VV. Myeloid cell death associated with Toll-like receptor 7/8-mediated inflammatory response. Implication of ASK1, HIF-1 alpha, IL-1 beta and TNF-alpha. Mol Immunol 2010;48:240–7 [DOI] [PubMed] [Google Scholar]
- 61. Kotliar D, Lin AE, Logue J, Hughes TK, Khoury NM, Raju SS, Wadsworth MH, 2nd, Chen H, Kurtz JR, Dighero-Kemp B, Bjornson ZB, Mukherjee N, Sellers BA, Tran N, Bauer MR, Adams GC, Adams R, Rinn JL, Mele M, Schaffner SF, Nolan GP, Barnes KG, Hensley LE, McIlwain DR, Shalek AK, Sabeti PC, Bennett RS. Single-cell profiling of Ebola virus disease in vivo reveals viral and host dynamics. Cell 2020;183:1383–401.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest 2000;80:171–86 [DOI] [PubMed] [Google Scholar]
- 63. Bradfute SB, Swanson PE, Smith MA, Watanabe E, McDunn JE, Hotchkiss RS, Bavari S. Mechanisms and consequences of Ebola virus-induced lymphocyte apoptosis. J Immunol 2010;184:327–35 [DOI] [PubMed] [Google Scholar]
- 64. Baize S, Leroy EM, Mavoungou E, Fisher-Hoch SP. Apoptosis in fatal Ebola infection. Does the virus toll the bell for immune system? Apoptosis 2000;5:5–7 [DOI] [PubMed] [Google Scholar]
- 65. Nicholas VV, Rosenke R, Feldmann F, Long D, Thomas T, Scott DP, Feldmann H, Marzi A. Distinct biological phenotypes of Marburg and Ravn virus infection in macaques. J Infect Dis 2018;218:S458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Riabchikova EI, Kolesnikova LV, Rassadkin Iu N. [Microscopic study of species specific features of hemostatic impairment in Ebola virus infected monkeys]. Vestn Ross Akad Med Nauk 1998;3:51–5 [PubMed] [Google Scholar]
- 67. Mezoh G, Crowther NJ. Endothelial dysfunction as a primary consequence of SARS-CoV-2 infection. Adv Exp Med Biol 2021;1321:33–43 [DOI] [PubMed] [Google Scholar]
- 68. Adu-Gyamfi E, Johnson KA, Fraser ME, Scott JL, Soni SP, Jones KR, Digman MA, Gratton E, Tessier CR, Stahelin RV. Host cell plasma membrane phosphatidylserine regulates the assembly and budding of Ebola virus. J Virol 2015;89:9440–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kyle JE, Burnum-Johnson KE, Wendler JP, Eisfeld AJ, Halfmann PJ, Watanabe T, Sahr F, Smith RD, Kawaoka Y, Waters KM, Metz TO. Plasma lipidome reveals critical illness and recovery from human Ebola virus disease. Proc Natl Acad Sci U S A 2019;116:3919–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang L, Richard AS, Jackson CB, Ojha A, Choe H. Phosphatidylethanolamine and phosphatidylserine synergize to enhance GAS6/AXL-mediated virus infection and efferocytosis. J Virol 2020;95:e02079-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Richard AS, Zhang A, Park SJ, Farzan M, Zong M, Choe H. Virion-associated phosphatidylethanolamine promotes TIM1-mediated infection by Ebola, dengue, and West Nile viruses. Proc Natl Acad Sci U S A 2015;112:14682–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smither SJ, O’Brien LM, Eastaugh L, Woolley T, Lever S, Fletcher T, Parmar K, Hunt BJ, Watts S, Kirkman E. Haemostatic changes in five patients infected with Ebola virus. Viruses 2019;11:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wilson AJ, Martin DS, Maddox V, Rattenbury S, Bland D, Bhagani S, Cropley I, Hopkins S, Mepham S, Rodger A, Warren S, Chowdary P, Jacobs M. Thromboelastography in the management of coagulopathy associated with Ebola virus disease. Clin Infect Dis 2016;62:610–2 [DOI] [PubMed] [Google Scholar]
- 74. Bhatnagar P, Sreekanth GP, Murali-Krishna K, Chandele A, Sitaraman R. Dengue virus non-structural protein 5 as a versatile, multi-functional effector in host-pathogen interactions. Front Cell Infect Microbiol 2021;11:574067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nanaware N, Banerjee A, Mullick Bagchi S, Bagchi P, Mukherjee A. Dengue virus infection: a tale of viral exploitations and host responses. Viruses 2021;13:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guo C, Zhou Z, Wen Z, Liu Y, Zeng C, Xiao D, Ou M, Han Y, Huang S, Liu D, Ye X, Zou X, Wu J, Wang H, Zeng EY, Jing C, Yang G. Global epidemiology of dengue outbreaks in 1990-2015: a systematic review and meta-analysis. Front Cell Infect Microbiol 2017;7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leong AS, Wong KT, Leong TY, Tan PH, Wannakrairot P. The pathology of dengue hemorrhagic fever. Semin Diagn Pathol 2007;24:227–36 [DOI] [PubMed] [Google Scholar]
- 78. Martins Sde T, Silveira GF, Alves LR, Duarte dos Santos CN, Bordignon J. Dendritic cell apoptosis and the pathogenesis of dengue. Viruses 2012;4:2736–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Uno N, Ross TM. Dengue virus and the host innate immune response. Emerg Microbes Infect 2018;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sun P, Kochel TJ. The battle between infection and host immune responses of dengue virus and its implication in dengue disease pathogenesis. Sci World J 2013;2013:843469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg 2006;100:608–14 [DOI] [PubMed] [Google Scholar]
- 82. Castillo JA, Urcuqui-Inchima S. Mechanisms of monocyte cell death triggered by dengue virus infection. Apoptosis 2018;23:576–86 [DOI] [PubMed] [Google Scholar]
- 83. Ghosh Roy S, Sadigh B, Datan E, Lockshin RA, Zakeri Z. Regulation of cell survival and death during flavivirus infections. World J Biol Chem 2014;5:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pan Y, Cheng A, Wang M, Yin Z, Jia R. The dual regulation of apoptosis by flavivirus. Front Microbiol 2021;12:654494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Okamoto T, Suzuki T, Kusakabe S, Tokunaga M, Hirano J, Miyata Y, Matsuura Y. Regulation of apoptosis during flavivirus infection. Viruses 2017;9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marianneau P, Flamand M, Courageot MP, Deubel V, Despres P. [Apoptotic cell death in response to dengue virus infection: what are the consequences of viral pathogenesis?]. Ann Biol Clin (Paris) 1998;56:395–405 [PubMed] [Google Scholar]
- 87. Kumar R, Mehta D, Mishra N, Nayak D, Sunil S. Role of host-mediated post-translational modifications (PTMs) in RNA virus pathogenesis. Int J Mol Sci 2020;22:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Castano-Rodriguez C, Honrubia JM, Gutierrez-Alvarez J, Sola I, Enjuanes L. Viral PDZ binding motifs influence cell behavior through the interaction with cellular proteins containing PDZ domains. Methods Mol Biol 2021;2256:217–36 [DOI] [PubMed] [Google Scholar]
- 89. Green AM, Beatty PR, Hadjilaou A, Harris E. Innate immunity to dengue virus infection and subversion of antiviral responses. J Mol Biol 2014;426:1148–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Courageot MP, Catteau A, Despres P. Mechanisms of dengue virus-induced cell death. Adv Virus Res 2003;60:157–86 [DOI] [PubMed] [Google Scholar]
- 91. Lei HY, Yeh TM, Liu HS, Lin YS, Chen SH, Liu CC. Immunopathogenesis of dengue virus infection. J Biomed Sci 2001;8:377–88 [DOI] [PubMed] [Google Scholar]
- 92. Martina BE. Dengue pathogenesis: a disease driven by the host response. Sci Prog 2014;97:197–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gulati A. Vascular endothelium and hypovolemic shock. Curr Vasc Pharmacol 2016;14:187–95 [DOI] [PubMed] [Google Scholar]
- 94. Chao CH, Wu WC, Lai YC, Tsai PJ, Perng GC, Lin YS, Yeh TM. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog 2019;15:e1007625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brenes H, Loria GD, Lomonte B. Potent virucidal activity against Flaviviridae of a group IIA phospholipase A2 isolated from the venom of Bothrops asper. Biologicals 2020;63:48–52 [DOI] [PubMed] [Google Scholar]
- 96. Yang X, Cheng X, Tang Y, Qiu X, Wang Z, Fu G, Wu J, Kang H, Wang J, Wang H, Chen F, Xiao X, Billiar TR, Lu B. The role of type 1 interferons in coagulation induced by gram-negative bacteria. Blood 2020;135:1087–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tang N. Response to “Reply to Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy.” J Thromb Haemost 2020;18:1520–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, Wang F, Li G, Li Y, Xing L, Peng L, Yang M, Cao M, Zheng H, Wu W, Zou R, Li D, Xu Z, Wang H, Zhang M, Zhang Z, Gao GF, Jiang C, Liu L, Liu Y. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol 2020;146:119–27.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fan BE. Hematologic parameters in patients with COVID-19 infection: a reply. Am J Hematol 2020;95:E215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ravindra PV, Tiwari AK, Ratta B, Chaturvedi U, Palia SK, Chauhan RS. Newcastle disease virus-induced cytopathic effect in infected cells is caused by apoptosis. Virus Res 2009;141:13–20 [DOI] [PubMed] [Google Scholar]
- 102. Lopez-Herrera A, Ruiz-Saenz J, Goez YP, Zapata W, Velilla PA, Arango AE, Urcuqui-Inchima S. Apoptosis as pathogenic mechanism of infection with vesicular stomatitis virus. Biocell 2009;33:121–32 [PubMed] [Google Scholar]
- 103. Lecoeur H, Melki MT, Saidi H, Gougeon ML. Analysis of apoptotic pathways by multiparametric flow cytometry: application to HIV infection. Methods Enzymol 2008;442:51–82 [DOI] [PubMed] [Google Scholar]
- 104. Shiratsuchi A, Nakanishi Y. [Elimination of influenza virus-infected cells by phagocytosis]. Yakugaku Zasshi 2006;126:1245–51 [DOI] [PubMed] [Google Scholar]
- 105. Rode M, Balkow S, Sobek V, Brehm R, Martin P, Kersten A, Dumrese T, Stehle T, Mullbacher A, Wallich R, Simon MM. Perforin and Fas act together in the induction of apoptosis, and both are critical in the clearance of lymphocytic choriomeningitis virus infection. J Virol 2004;78:12395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B, Li J, Wang Z, Wu W, Wu M, Li W, Li L, Cai Y, Bosco B, Zhong A, Liu X, Lv T, Gan Z, Chen G, Pan Y, Liu C, Zhang K, Xu X, Wang C, Wang Q. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood 2020;136:755–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Selvaraj V, Dapaah-Afriyie K, Finn A, Flanigan TP. Short-term dexamethasone in sars-CoV-2 patients. R I Med J (2013) 2020;103:39–43 [PubMed] [Google Scholar]
- 109. Antwi-Amoabeng D, Kanji Z, Ford B, Beutler BD, Riddle MS, Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review. J Med Virol 2020;92:2516–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Barberis E, Timo S, Amede E, Vanella VV, Puricelli C, Cappellano G, Raineri D, Cittone MG, Rizzi E, Pedrinelli AR, Vassia V, Casciaro FG, Priora S, Nerici I, Galbiati A, Hayden E, Falasca M, Vaschetto R, Sainaghi PP, Dianzani U, Rolla R, Chiocchetti A, Baldanzi G, Marengo E, Manfredi M. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int J Mol Sci 2020;21:8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pungercar J, Bihl F, Lambeau G, Krizaj I. What do secreted phospholipases A2 have to offer in combat against different viruses up to SARS-CoV-2? Biochimie 2021;189:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li Y, Jiang Y, Zhang Y, Li N, Yin Q, Liu L, Lv X, Liu Y, Li A, Fang B, Li J, Ye H, Yang G, Cui X, Liu Y, Qu Y, Li C, Li J, Li D, Gai Z, Wang S, Zhan F, Liang M. Abnormal upregulation of cardiovascular disease biomarker PLA2G7 induced by proinflammatory macrophages in COVID-19 patients. Sci Rep 2021;11:6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Roy Wong LY, Zheng J, Wilhelmsen K, Li K, Ortiz ME, Schnicker NJ, Pezzulo AA, Szachowicz PJ, Klumpp K, Aswad F, Rebo J, Narumiya S, Murakami M, Meyerholz DK, Fortney K, McCray PB, Perlman S. Eicosanoid signaling as a therapeutic target in middle-aged mice with severe COVID-19. bioRxiv 2021. DOI: 10.1101/2021.04.20.440676 [DOI] [Google Scholar]
- 114. Hoxha M. What about COVID-19 and arachidonic acid pathway? Eur J Clin Pharmacol 2020;76:1501–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase JM, Burudpakdee C, Lee JH, Jasen C, Balamuth F, Barrett DM, Banwell B, Bernt KM, Blatz AM, Chiotos K, Fisher BT, Fitzgerald JC, Gerber JS, Gollomp K, Gray C, Grupp SA, Harris RM, Kilbaugh TJ, Odom John AR, Lambert MP, Liebling EJ, Paessler M, Petrosa W, Phillips CA, Reilly AF, Romberg N, Seif AE, Sesok-Pizzini D, Sullivan K, Vardaro J, Behrens EM, Teachey DT, Bassiri H. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest 2020;130:5967–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, Ansusinha E, Hahn A, Hamdy R, Harik N, Hanisch B, Jantausch B, Koay A, Steinhorn R, Newman K, Wessel D. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr 2020;223:199–203.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Latimer G, Corriveau C, DeBiasi RL, Jantausch B, Delaney M, Jacquot C, Bell M, Dean T. Cardiac dysfunction and thrombocytopenia-associated multiple organ failure inflammation phenotype in a severe paediatric case of COVID-19. Lancet Child Adolesc Health 2020;4:552–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, Heidemann SM, Kleinman LC, Sen AI, Hall MW, Priestley MA, McGuire JK, Boukas K, Sharron MP, Burns JP. International COVID-19 PICU Collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020;174:868–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gotzinger F, Santiago-Garcia B, Noguera-Julian A, Lanaspa M, Lancella L, Calo Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L’Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglic S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M. ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020;4:653–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. CDC, 2020, https://www.cdc.gov/mis-c/hcp/
- 122. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS, Jr., Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG, Overcoming COVID-19 Investigators, the CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H, New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020;142:429–36 [DOI] [PubMed] [Google Scholar]
- 125. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998;31:1–9 [DOI] [PubMed] [Google Scholar]
- 126. Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood 1996;87:1179–87 [PubMed] [Google Scholar]
- 127. Harahsheh AS, Dahdah N, Newburger JW, Portman MA, Piram M, Tulloh R, McCrindle BW, de Ferranti SD, Cimaz R, Truong DT, Burns JC. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr 2020;222:261–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Group WHOREAfC-TW Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, Savovic J, Tierney J, Baron G, Benbenishty JS, Berry LR, Broman N, Cavalcanti AB, Colman R, De Buyser SL, Derde LPG, Domingo P, Omar SF, Fernandez-Cruz A, Feuth T, Garcia F, Garcia-Vicuna R, Gonzalez-Alvaro I, Gordon AC, Haynes R, Hermine O, Horby PW, Horick NK, Kumar K, Lambrecht BN, Landray MJ, Leal L, Lederer DJ, Lorenzi E, Mariette X, Merchante N, Misnan NA, Mohan SV, Nivens MC, Oksi J, Perez-Molina JA, Pizov R, Porcher R, Postma S, Rajasuriar R, Ramanan AV, Ravaud P, Reid PD, Rutgers A, Sancho-Lopez A, Seto TB, Sivapalasingam S, Soin AS, Staplin N, Stone JH, Strohbehn GW, Sunden-Cullberg J, Torre-Cisneros J, Tsai LW, van Hoogstraten H, van Meerten T, Veiga VC, Westerweel PE, Murthy S, Diaz JV, Marshall JC, Sterne JAC. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 2021;326:499–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, Nicastri E, Tzavara V, Kostis E, Dagna L, Koufargyris P, Dimakou K, Savvanis S, Tzatzagou G, Chini M, Cavalli G, Bassetti M, Katrini K, Kotsis V, Tsoukalas G, Selmi C, Bliziotis I, Samarkos M, Doumas M, Ktena S, Masgala A, Papanikolaou I, Kosmidou M, Myrodia DM, Argyraki A, Cardellino CS, Koliakou K, Katsigianni EI, Rapti V, Giannitsioti E, Cingolani A, Micha S, Akinosoglou K, Liatsis-Douvitsas O, Symbardi S, Gatselis N, Mouktaroudi M, Ippolito G, Florou E, Kotsaki A, Netea MG, Eugen-Olsen J, Kyprianou M, Panagopoulos P, Dalekos GN, Giamarellos-Bourboulis EJ. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021;27:1752–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, Nicastri E, Tzavara V, Kostis E, Dagna L, Koufargyris P, Dimakou K, Savvanis S, Tzatzagou G, Chini M, Cavalli G, Bassetti M, Katrini K, Kotsis V, Tsoukalas G, Selmi C, Bliziotis I, Samarkos M, Doumas M, Ktena S, Masgala A, Papanikolaou I, Kosmidou M, Myrodia DM, Argyraki A, Cardellino CS, Koliakou K, Katsigianni EI, Rapti V, Giannitsioti E, Cingolani A, Micha S, Akinosoglou K, Liatsis-Douvitsas O, Symbardi S, Gatselis N, Mouktaroudi M, Ippolito G, Florou E, Kotsaki A, Netea MG, Eugen-Olsen J, Kyprianou M, Panagopoulos P, Dalekos GN, Giamarellos-Bourboulis EJ. Author correction: early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021;27:1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, Arduino R, Fomina D, Bogdanov R, Stepanenko T, Ruiz-Seco P, Gonzalez-Garcia A, Chen Y, Li Y, Whelan S, Noviello S, Investigators C-C. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA 2021;326:230–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Neys SFH, Hendriks RW, Corneth OBJ. Targeting Bruton’s tyrosine kinase in inflammatory and autoimmune pathologies. Front Cell Dev Biol 2021;9:668131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Guimaraes PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, Kalil Filho R, Junior VM, Soeiro AM, Tognon AP, Veiga VC, Martins PA, Moia DDF, Sampaio BS, Assis SRL, Soares RVP, Piano LPA, Castilho K, Momesso R, Monfardini F, Guimaraes HP, Ponce de, Leon D, Dulcine M, Pinheiro MRT, Gunay LM, Deuring JJ, Rizzo LV, Koncz T, Berwanger O. STOP-COVID Trial Investigators. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;385:406–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Menschikowski M, Hagelgans A, Siegert G. Secretory phospholipase A2 of group IIA: is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases. Prostaglandins Other Lipid Mediat 2006;79:1–33 [DOI] [PubMed] [Google Scholar]
- 135. Cohen SP, Hooten WM, Phillips CR. Pain management during COVID-19 and steroids: striking a balance. Pain Med 2020;21:1731–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Fernandez Cruz A, Ruiz-Antoran B, Munoz Gomez A, Sancho Lopez A, Mills Sanchez P, Centeno Soto GA, Blanco Alonso S, Javaloyes Garachana L, Galan Gomez A, Valencia Alijo A, Gomez Irusta J, Payares-Herrera C, Morras Torre I, Sanchez Chica E, Delgado Tellez, de Cepeda L, Callejas Diaz A, Ramos Martinez A, Munez Rubio E, Avendano-Sola C, Puerta de Hierro COVID-19 Study Group. Impact of glucocorticoid treatment in Sars-Cov-2 infection mortality: a retrospective controlled cohort study. Antimicrob Agents Chemother 2020;64:e01168–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gazzaruso C, Carlo Stella N, Mariani G, Tamburlini A, Garini P, Freddi E, Ravetto C, Coppola A, Gallotti P. Impact of anti-rheumatic drugs and steroids on clinical course and prognosis of COVID-19. Clin Rheumatol 2020. DOI: 10.1007/s10067-020-05239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kolilekas L, Loverdos K, Giannakaki S, Vlassi L, Levounets A, Zervas E, Gaga M. Can steroids reverse the severe COVID-19 induced ‘cytokine storm’? J Med Virol 2020. DOI: 10.1002/jmv.26165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. RECOVERY Trial, 2020, https://rebelem.com/the-recovery-trial-dexamethasone-for-covid-19
- 140. Yao T-C, Huang Y-W, Chang S-M, Tsai S-Y, Wu AC, Tsai H-J. Association between oral corticosteroid bursts and severe adverse events. Ann Intern Med 2020;173:325–30 [DOI] [PubMed] [Google Scholar]
- 141. Kim GW, Lee NR, Pi RH, Lim YS, Lee YM, Lee JM, Jeong HS, Chung SH. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res 2015;38:575–84 [DOI] [PubMed] [Google Scholar]
- 142. Infliximab, 2020, https://www.drugs.com/monograph/infliximab-infliximab-dyyb.html
- 143. Fraser H, Hislop C, Christie RM, Rick HL, Reidy CA, Chouinard ML, Eacho PI, Gould KE, Trias J. Varespladib (A-002), a secretory phospholipase A2 inhibitor, reduces atherosclerosis and aneurysm formation in ApoE-/- mice. J Cardiovasc Pharmacol 2009;53:60–5 [DOI] [PubMed] [Google Scholar]
- 144. Zeiher BG, Steingrub J, Laterre PF, Dmitrienko A, Fukiishi Y, Abraham E. EZZI Study Group. LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit Care Med 2005;33:1741–8 [DOI] [PubMed] [Google Scholar]
- 145. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020;323:2052–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Albano E. Seek and hide phosphatidylserine: a new approach to prevent hepatic ischemia/reperfusion injury. Gastroenterology 2007;133:713–6 [DOI] [PubMed] [Google Scholar]
- 149. Hale SL, Allison AC, Kloner RA. Diannexin reduces no-reflow after reperfusion in rabbits with large ischemic myocardial risk zones. Cardiovasc Ther 2011;29:e42–52 [DOI] [PubMed] [Google Scholar]
- 150. Hashimoto K, Kim H, Oishi H, Chen M, Iskender I, Sakamoto J, Ohsumi A, Guan Z, Hwang D, Waddell TK, Cypel M, Liu M, Keshavjee S. Annexin V homodimer protects against ischemia reperfusion-induced acute lung injury in lung transplantation. J Thorac Cardiovasc Surg 2016;151:861–9 [DOI] [PubMed] [Google Scholar]
- 151. Powell JT, Tsapepas DS, Martin ST, Hardy MA, Ratner LE. Managing renal transplant ischemia reperfusion injury: novel therapies in the pipeline. Clin Transplant 2013;27:484–91 [DOI] [PubMed] [Google Scholar]
- 152. Teoh NC, Ajamieh H, Wong HJ, Croft K, Mori T, Allison AC, Farrell GC. Microparticles mediate hepatic ischemia-reperfusion injury and are the targets of Diannexin (ASP8597). PLoS ONE 2014; 9:e104376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wever KE, Wagener FA, Frielink C, Boerman OC, Scheffer GJ, Allison A, Masereeuw R, Rongen GA. Diannexin protects against renal ischemia reperfusion injury and targets phosphatidylserines in ischemic tissue. PLoS ONE 2011;6:e24276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cheng EY, Sharma VK, Chang C, Ding R, Allison AC, Leeser DB, Suthanthiran M, Yang H. Diannexin decreases inflammatory cell infiltration into the islet graft, reduces beta-cell apoptosis, and improves early graft function. Transplantation 2010;90:709–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shen XD, Ke B, Zhai Y, Tsuchihashi SI, Gao F, Duarte S, Coito A, Busuttil RW, Allison AC, Kupiec-Weglinski JW. Diannexin, a novel annexin V homodimer, protects rat liver transplants against cold ischemia-reperfusion injury. Am J Transplant 2007;7:2463–71 [DOI] [PubMed] [Google Scholar]