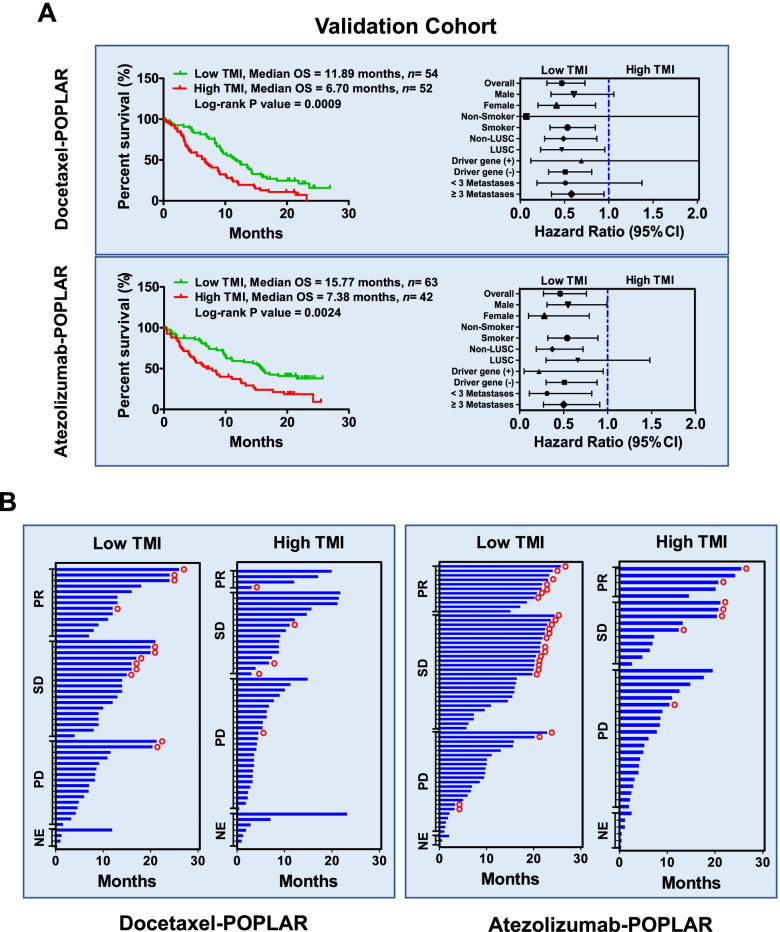
Fig. 3.
Validation of the effectiveness of the TMI in the validation cohort (POPLAR cohort). A Top Kaplan-Meier plots of OS in NSCLC patients receiving docetaxel after TMI stratification. For responders, the median OS duration was 11.89 months (n = 54); for non-responders, the median OS duration was 6.70 months (n = 52). The HRs of all patients and the corresponding subgroups are shown on the top right. Bottom Kaplan-Meier plots of OS in NSCLC patients receiving atezolizumab after TMI stratification. For responders, the median OS duration was 15.77 months (n = 63); for non-responders, the median OS duration was 7.38 months (n = 42). The HRs of all patients and the corresponding subgroups are shown on the lower right. B Left Evaluation of clinical efficacy in patients defined as having a low TMI and a high TMI after receiving docetaxel monotherapy. Right Evaluation of clinical efficacy in patients defined as having a low TMI and a high TMI after receiving atezolizumab monotherapy. The blue pillar represents the survival time for each patient; the red circle represents those still alive at the end of the follow-up