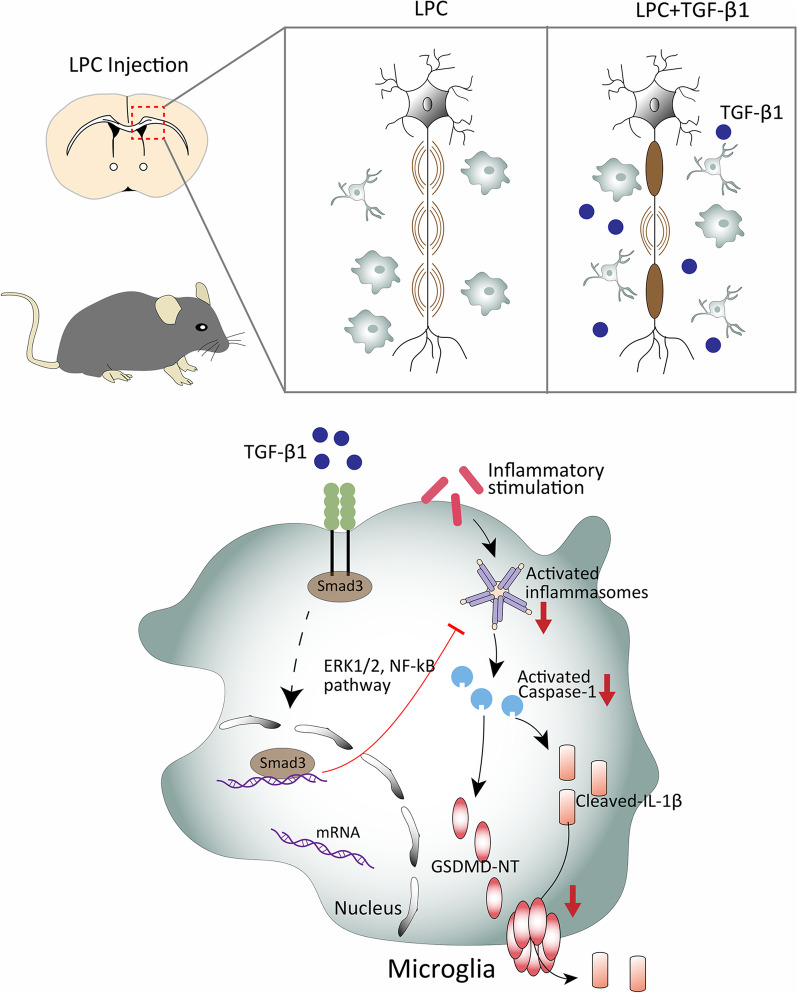
Fig. 8.
A schematic illustration showing the protective effects of TGF-β1 on LPC-induced demyelination and its underlying mechanisms. Brain stereotactic injection of LPC leads to evident demyelination and activated microglial pyroptosis in the corpus callosum of mice. Mechanistically, the external inflammatory stimulation activates the NLRP3 inflammasomes, which could cleave pro-caspase-1 to capspase-1-p20. Cleaved caspase-1 promotes the oligomerization of GSDMD to form membrane pores, as well as the maturation and release of IL-1β to extracellular space through membrane pores. Supplementary TGF-β1 binds to its receptor TGF-βRI/II and improves the translocation of its downstream molecule Smad3 into nuclei to exert subsequent functions. The upregulation of TGF-β1 signal pathway suppresses the process of pyroptosis in microglia by reducing the activated inflammasomes, cleaved-GSDMD, and IL-1β through ERK1/2 and NF-κB pathways. In brief, TGF-β1 could alleviate the neuroinflammation by reducing pro-inflammatory pyropsis of microglia and ultimately protect against demyelinating injury and cognitive disorder in LPC-induced demyelinating model