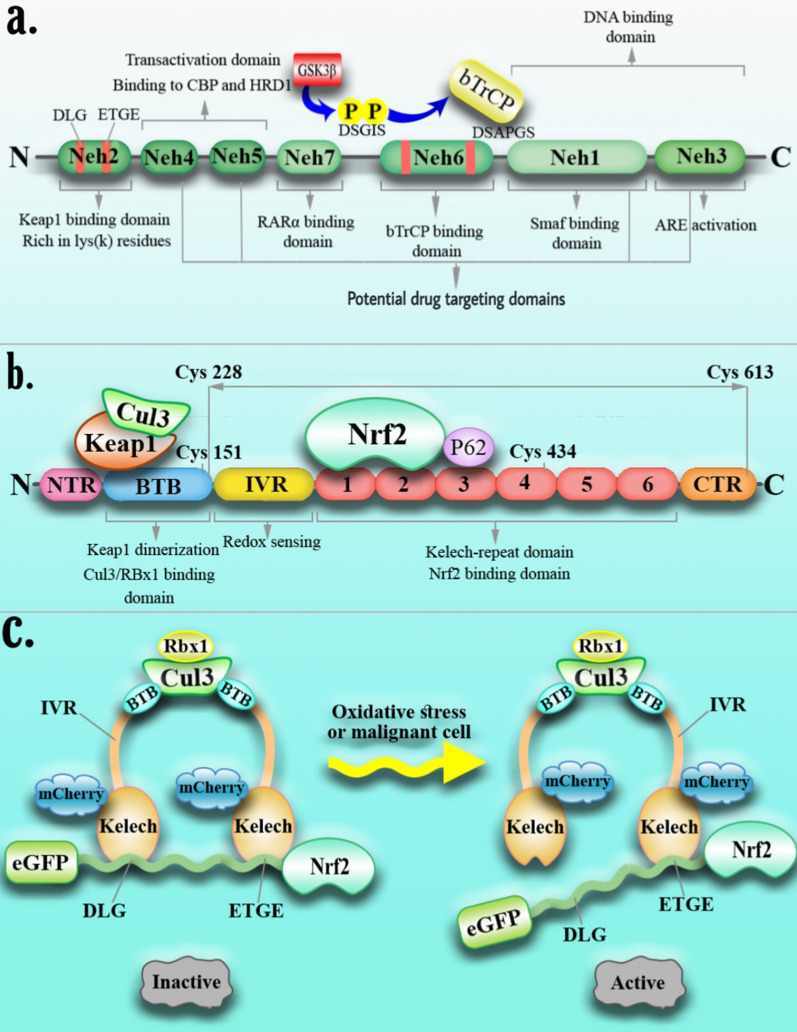
Fig. 1.
Interaction of Keap1 and Nrf2. Keap1 (an Nrf2 inhibitor) has three major domains: BTB, IVR, and Kelch. The BTB domain is involved in Keap1 dimerization, CUL3 binding, and ROS sensing by C151 (Cys). The Kelch domain consists of six Kelch repeats, which bind to Nrf2 and P65 proteins. The IVR domain is also a redox-sensing domain (a). Nrf2 has seven domains; Neh2 is the Keap1 binding domain through DLG and ETGE motif interaction. Neh4, 5, 3 domains are critical for transactivation. Moreover, Nrf2, through the Neh3 domain, binds to the ARE region of the chromosome. The Neh6 domain has serine (S) residues in the DSGIS motif. GSK3β phosphorylates this motif and supports recognition by βTrCP, and Neh1 interacts with small MAF proteins (b). Under normal conditions, Nrf2 has been suppressed through binding to the Kelch domain of Keap1, but in the presence of ROS or malignant cells, the release of Nrf2 from Keap1 occurs, and the transfer of Nrf2 to the nucleus alters the pattern of gene expression (c)