Figure EV1. (Related to Fig 1). LAQ partially alleviated phenotypes in PLP‐150Q mice.
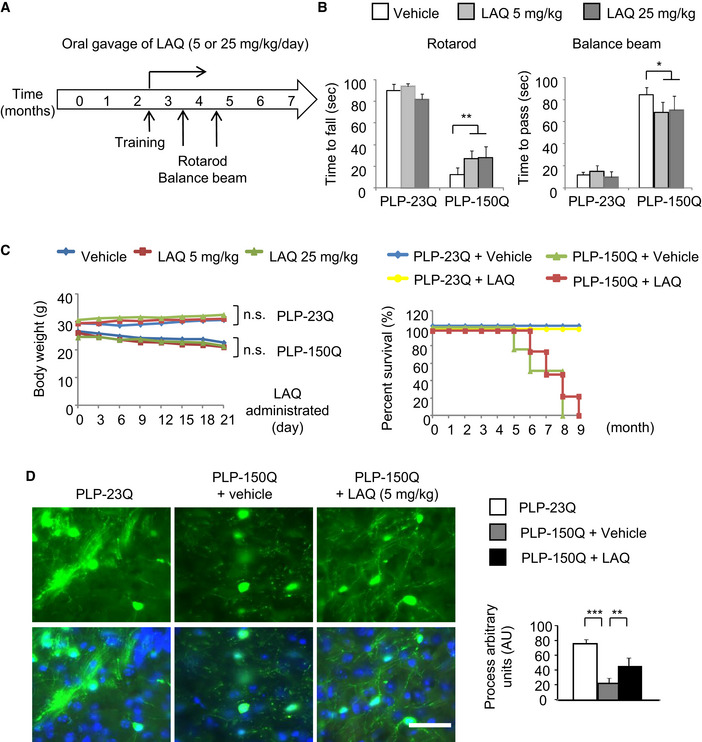
- Schematic of study design. PLP‐150Q mice and the control PLP‐23Q mice at the age of 3 months were orally administrated with LAQ (5 or 25 mg/kg/day as indicated in figure) for 2 months. The brain tissues were collected for pathological examination after behavioral studies.
- Rotarod performance and balance beam tests showed severe motor impairment in PLP‐150Q mice. Administrating 5 or 25 mg/kg LAQ could alleviate the impairment. One‐way ANOVA followed with Tukey's test. **P = 0.00735; *P = 0.04086; n = 12 mice per group for rotarod perform test and n = 6 mice per group for balance beam test. Data are presented as mean ± SEM.
- The PLP‐150Q mice show reduced body weight and early death, which were not altered by the treatment with 5 or 25 mg/kg LAQ for 2 months. n = 12 mice each group, which were treated with LAQ at 3 months of age. Data are mean ± SEM.
- PLP‐150Q/GFP mice show GFP labeling of oligodendrocyte processes, which are reduced in the corpus callosum when compared with PLP‐23Q/GFP mice and were increased after 5 mg/kg LAQ treatment for 2 months. Quantitative analysis of the number of GFP‐positive oligodendrocytes and process length was shown under the micrographs. n = 3 mice in each group. Process length was presented in a.u. One‐way ANOVA followed with Tukey's test. ***P = 3.92 × 10−5; **P = 0.00187. Data were mean ± SEM. Scale bar: 40 μm.
