Figure EV3. (Related to Figs 2 and 3). The anti‐pMYRF (Ser259) antibody recognized phosphor‐Serine 259 in N‐terminal MYRF.
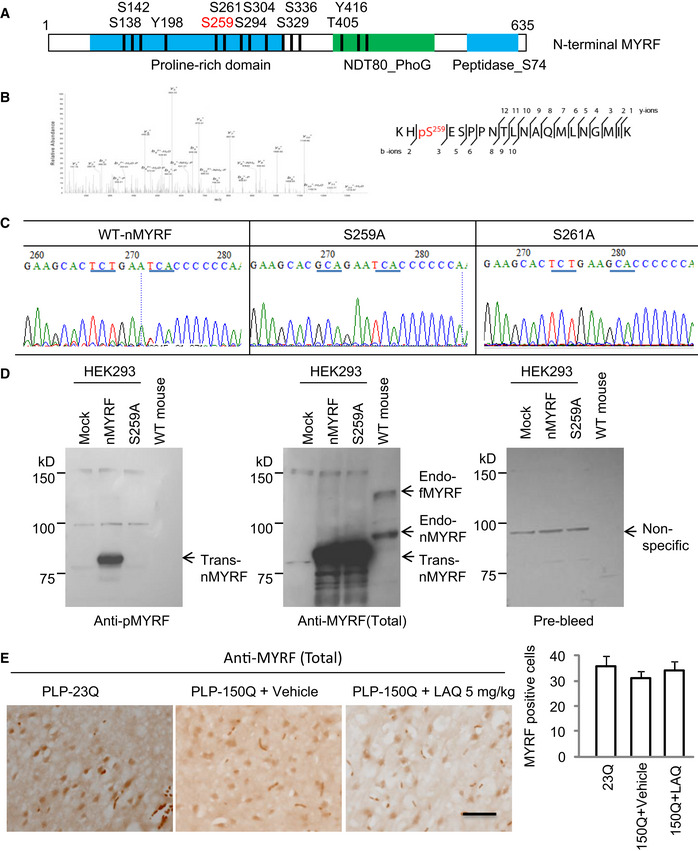
- Predicted potential phosphorylation sites in mouse N‐terminal MYRF (nMYRF). S259 (red) is a top candidate.
- Mass spectrometry of the immunoprecipitated MYRF revealed an enrichment phosphorylated signal at pS259.
- The sequences of mutant MYRF S259A and S261A verified Ser259 substitution.
- Western blotting of HEK293 cell expressing wild‐type N‐terminal MYRF (nMYRF), S259A or S261A cDNAs, and mouse tissues showing that replacing Ser259 with alanine can eliminate the labeling of MYRF by antibody to Ser259. The samples were also probed with antibody to total MYRF (Sigma, HAP018310) and pre‐immune serum. endo‐fMYRF: endogenous full‐length MYRF; endo‐nMYRF: endogenous N‐terminal MYRF; trans‐MYRF: transfected MYRF.
- The anti‐MYRF (Sigma, HAP018310) immunohistochemical staining of the corpus callosum of PLP‐23Q and PLP‐150Q mice showing no significant effect on the total MYRF expression by LAQ (5 mg/kg) treatment. n = 3 mice in each group. Scale bar: 10 μm. Quantitative analysis of the MYRF‐positive cells per field (40×) was presented on the right. Data were mean ± SEM.
