Figure 2. There is a single binding site on the CaMKII kinase domain.
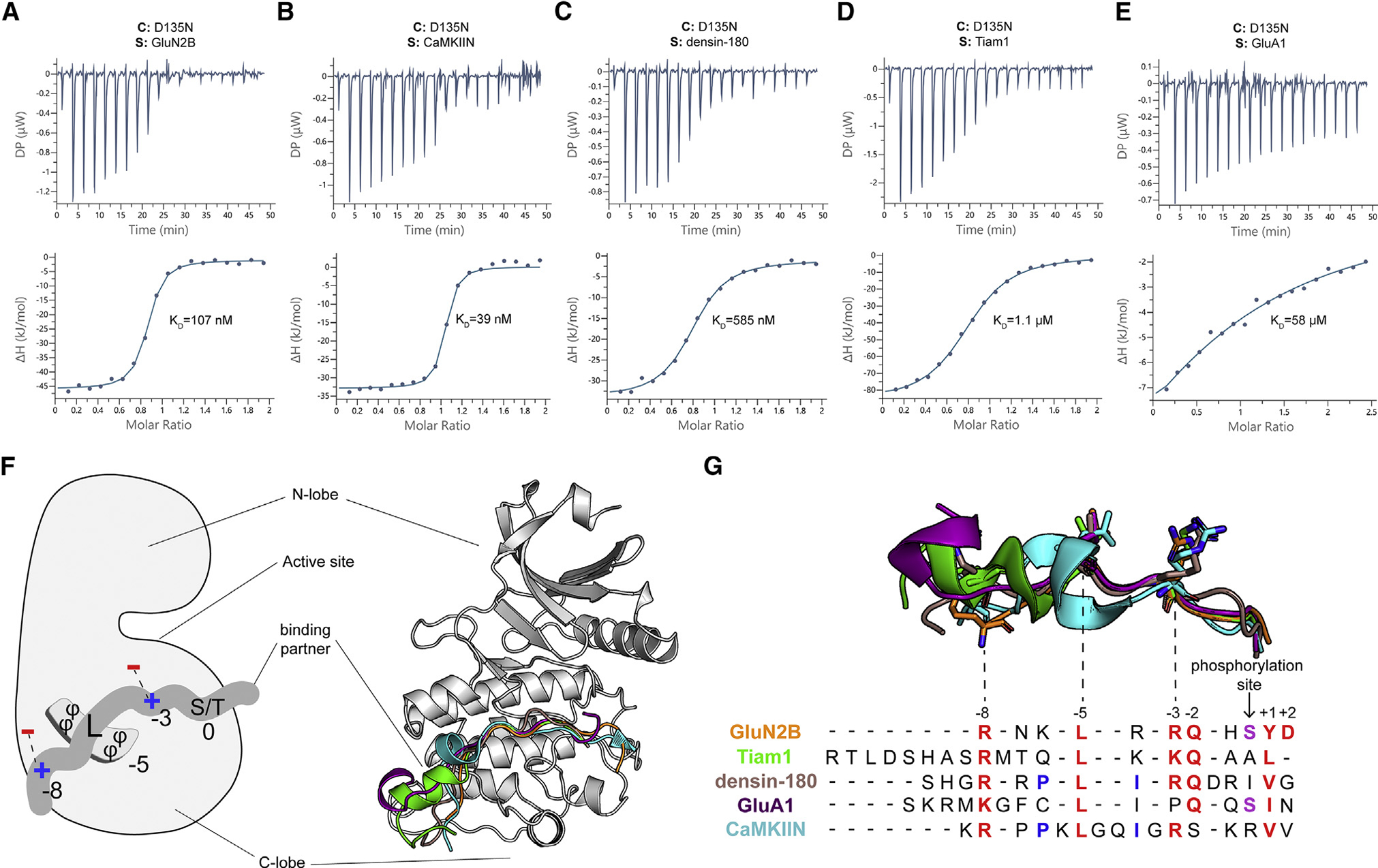
(A–E) Representative ITC measurements of D135N kinase domain binding to peptide partners. Contents of the cell (C) and syringe (S) are indicated. The mean Kd value from two independent measurements is labeled.
(F) Left: schematic of the interactions with binding partners. The phi symbol indicates hydrophobic residues. Right: overlay of five cocrystal structures, peptides shown as cartoon in corresponding colors seen in key to the right.
(G) The sequence alignment of CaMKII binding partners. Binding partner position numbering is based on the prototypical GluN2B substrate with the phosphorylation site set to zero. Aligned peptide structures are shown above for reference. Conserved residues are colored red. Phosphorylatable residues at the phosphorylation site are colored purple. Residues involved in a docking event with W214 are colored blue.
