Figure 4. Electrostatic interactions with a basic residue at the −3 position facilitate high-affinity binding.
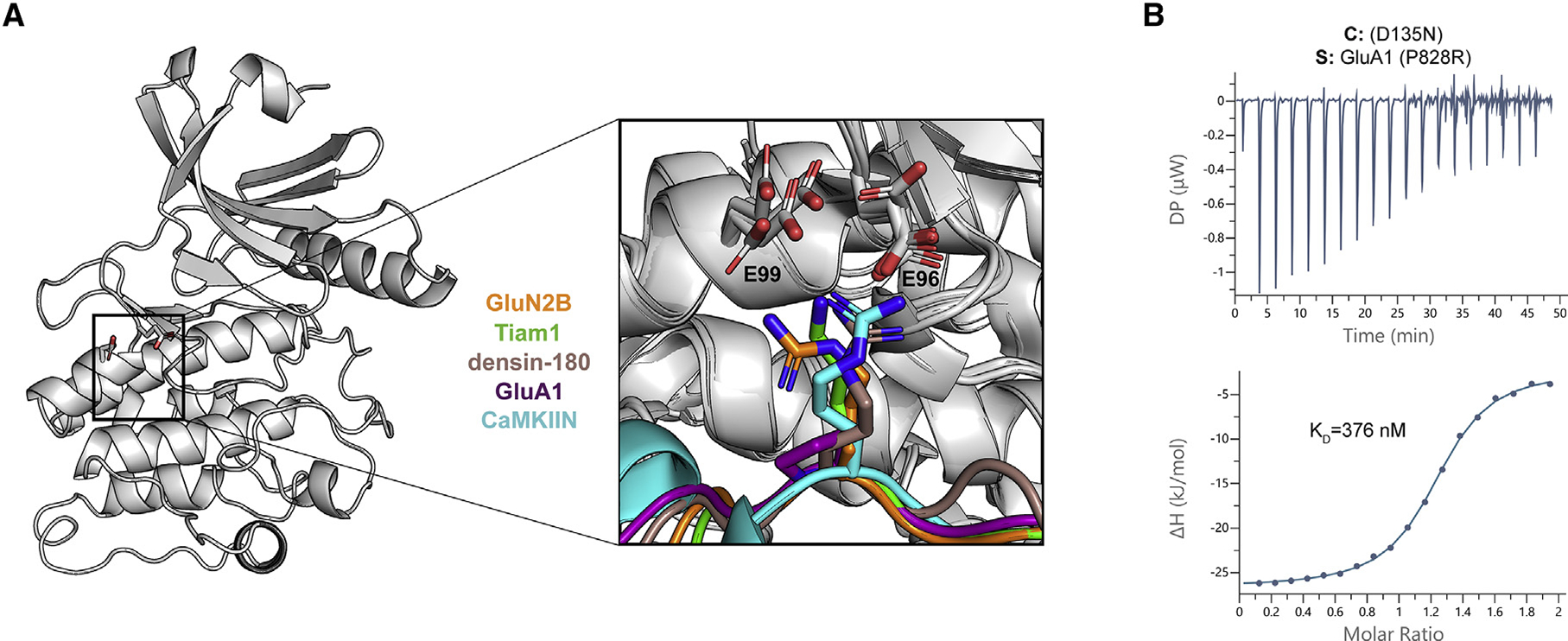
(A) CaMKII kinase domain shown as a cartoon; the E96 and E99 residues are shown as sticks. A magnified view of all five co-crystal structures is overlaid to highlight the basic residue at the −3 position (except for GluA1 in purple) interacting with the two glutamic acids in the kinase domain.
(B) ITC data for the D135N CaMKII kinase domain (cell) and GluA1 with P828R mutation (syringe). The mean Kd value is from two independent measurements.
