Table 1.
Stereochemical control in enone reduction and introduction of the C12 stereogenic center
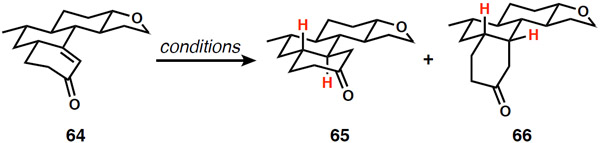
| |||
|---|---|---|---|
| Entry | Conditions | Yielda | 65:66 (trans:cis) |
| 1 | Li, NH3, THF, −40 °C | 64% | 1:2 |
| 2 | Na, NH3, THF, −78 °C | 85% | 1:1 |
| 3 | K, NH3, THF, −78 °C | 82% | 1:1 |
| 4 | K, t-BuOH, NH3, THF, −78 °C | 80% | 1:3 |
| 5 | Karstedt’s catb, Et3SiHc | 92% | 1:5 |
| 6 | t-BuCu, DIBAL-H, HMPA, THF | 86% | 1:>20 |
| 7 | H2, Pd/C, EtOAc | 94% | 6:1 |
| 8 | H2, Rh/alumina, EtOAcd | 93% | 8:1 |
| 9 | H2, Rh/C, EtOAcd | 93% | 15:1 |
| 10 | H2, Pt/C, EtOAc | trace | nd |
| 11 | H2, Ru/C, EtOAc | trace | 10:1 |
Yield of purified material after column chromatography
Karstedt’s Pt hydrosilylation catalyst
followed by TBAF, THF to convert enoxysilane to ketone
followed by PCC, Celite, CH2Cl2 to reoxidize some carbinol.
