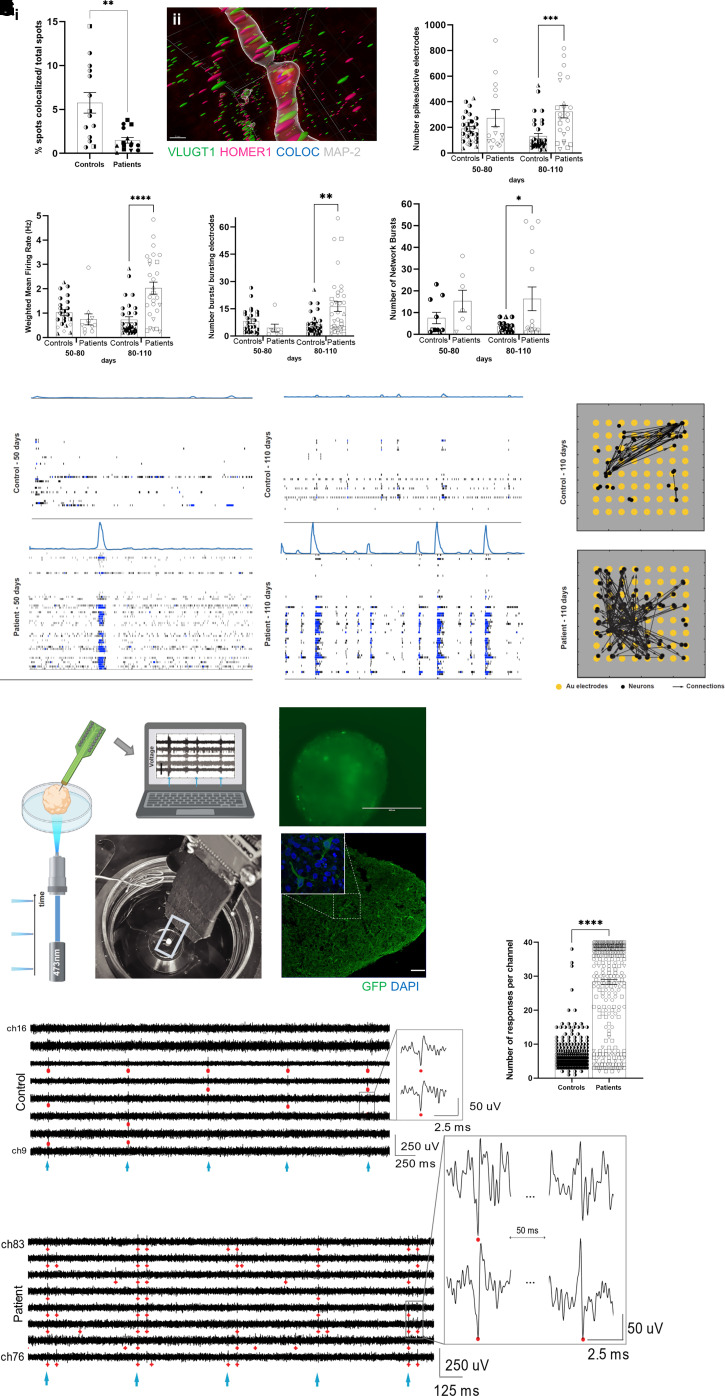
Figure 3.
Functional characterization of FCD cortical organoids. [A(i)] Reduction of co-localized synaptic puncta, using VGLUT1 (presynaptic antibody) and HOMER1 (postsynaptic), in 90- and 150-day-old organoids (n = 13 controls and n = 15 patients); 70 × 70 µm regions of interest per organoid; P = 0.0014. [A(ii)] Representative image of spot co-localization (blue sphere) using VGLUT1 (green sphere), HOMER1 (magenta sphere), and MAP2+ neurite (grey) from a 90-day-old control organoid. The image was obtained by using 3D imaging of cleared organoids for whole-mount immunofluorescence staining and Imaris software. (B–
E) Extracellular recordings using MEAs of 3-min intervals of spontaneous activity at different developmental stages (50–80 and 80–110 days) in control and FCD organoids. MEA analyses of FCD organoids with 80–110-day-old organoids revealed an increase in: (B) the number of spikes (P = 0.0002); (C) the mean firing rate (P < 0.0001); (D) the number of bursts (P = 0.0017); and (E) the number of spontaneous network bursts (P = 0.024). Each dot represents the result of a weekly measurement of a single well of an MEA plate (two to three organoids plated per well). Two independent experiments were conducted for each subject, with three technical replicates per subject in each experiment. (F) Raster plots of network spiking activity relative to 50-day-old (left) and 110-day-old (right) in WT83 control and F1 cortical organoids. (G) Effective connectivity inferred from MEA recordings of 110-old-day control (top) and F1 (bottom) cortical organoids reflect higher connectivity in the FCD organoids. Each visual map consists of a 1.2 × 1.2 mm MEA plate (grey area), an 8-by-8 array of micro-electrodes (yellow circles), and the estimated causal and direct connections (black arrows) between spike sorted neurons. The active neurons are represented by black dots; they are randomly distributed around their corresponding sensing electrode within a radius of 50 µm. The MEA results are reported as the mean ± SEM; statistical significance is based on two-way ANOVA and Bonferroni’s multiple comparisons test. (H–
K) Extracellular recordings using silicon high-density penetrating probes and stimulation via optogenetic actuation of CheRiff-expressing organoids. (H) Schematics and photograph of our setup for optogenetic stimulation with a 473-nm laser light and 3D extracellular recordings in organoids using penetrating high-density silicon microelectrodes. (I) Representative image of an FCD organoid transduced with DRH313-FCK-CheRiff-eGFP (top) and immunostaining (bottom) of 150-day-old organoid with GFP (green; indicating expression of the EGFP-CheRiff; scale bar = 100 μm) and DAPI (blue). (J) Representative recordings of optogenetic-evoked activity in control (top) and FCD (bottom) 150-day-old-organoids showing response in most of the considered channels. The cyan markers indicate pulses of 473-nm light (frequency of stimulation of 2 Hz). The red markers indicate detected spikes in the recorded activity (threshold of spike detection 5.5 × standard deviation). The insets show an enlarged view of neuronal spiking in response to optogenetic stimulation. Predominantly in FCD organoids, we observed double-spike event behaviour: the first spike followed by a second event of different polarity at a latency of ∼40–50 ms. FCD organoids presented (K) more optogenetic-elicited responses per channel than the controls (n = 365 controls and n = 758 patients) channels; all responding channels from all successful experiments were considered to be as independent variables. Extracellular recordings using silicon high-density probes results are presented as the mean ± SEM. Statistical significance based on one-sample t-test; *P < 0.05, **P < 0.001 and ****P < 0.0001. Controls: WT83 clone 1◑, clone 2◐; 4C clone 1◨, clone 2 ◧; 969 clone 1◮, clone 2 ◭, 121 clone 1 ◆ clone 2 ◇; patients: F1 clone 1●, clone 2 ○; F2 clone 1■, clone 2 □, F3 clone 1▲, clone 2 △, F4 clone 1▼, clone 2▽.