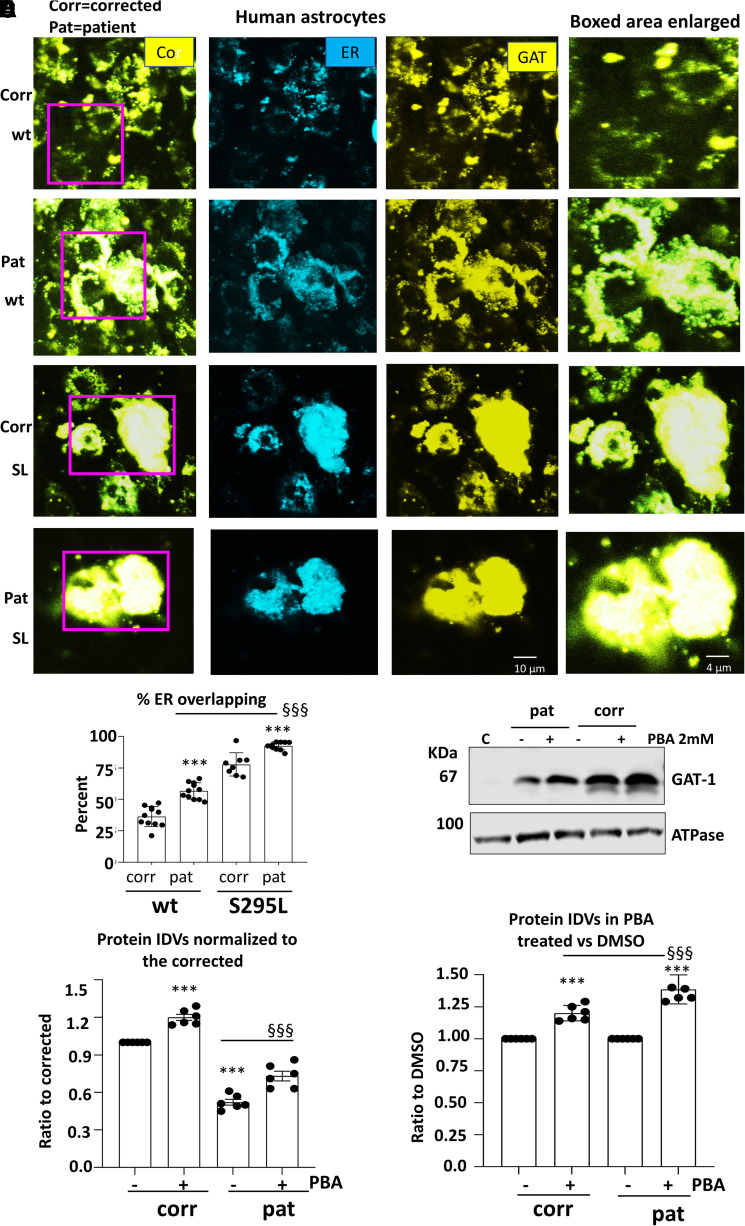
Figure 5.
The patient astrocytes caused ER retention of the wildtype and exacerbated the mutant GAT-1 ER retention while 4-phenylbutyrate acid (PBA) increased GAT-1 protein expression. (A, B) Human patient corrected (isogenic control, Corr) or uncorrected patient (Pat) astrocytes at Days 30–35 after differentiation from iPSCs were co-transfected with the endoplasmic reticulum (ER) marker ERCFP in combination with the wildtype or the mutant GAT-1YFP cDNAs (0.5 µg:0.5 µg per a 35 mm2 dish) for 48 h before confocal microscopy analysis. Confocal images were acquired in live astrocytes under 63X objective with zoom under 2.5. (A) Boxed regions were enlarged. (B) The graph represents the ER overlapping signal of GAT-1YFP analysed by Metamorph. (C, D) Total cell lysates from astrocytes cultured in 100 mm2 dishes treated with DMSO or PBA (2 mM) for 24 h and were analysed with SDS-PAGE. Membranes were blotted with a rabbit polyclonal atnti-GAT-1 antibody (C). The lysates of Chinese hamster ovary (CHO) cells were used as control. (D, E) The protein IDVs of the corrected or patient GAT-1 in human astrocytes were normalized to the corrected astrocytes treated with DMSO, the GAT-1 protein was normalized to its own internal control ATPase or GAPDH and then to the DMSO-treated corrected levels, which is arbitrarily taken as 1 (D) or its own genotype but DMSO treated, which is taken as 1 (E). Values were expressed as mean ± SEM. In B, N = 8–11 culture replicates. In C, D and E, N = 6 batch of cells. In B, ***P < 0.001 versus corrected. §§§P < 0.001 versus wt in patient cells. In D, ***P < 0.001 corrected untreated. §§§P < 0.001 versus patient untreated. In E, ***P < 0.001 versus its own untreated; §§§P < 0.001 versus corrected PBA treated, two-way analysis of variance followed by Bonferroni multiple comparison test.