Abstract
A nonenzymatic glucose sensor based on a disposable pencil graphite electrode (PGE) modified by copper nanoparticles [Cu(NP)] was prepared for the first time. The prepared Cu(NP) exhibited an absorption peak centered at ~562 nm using UV-visible spectrophotometry and an almost homogenous spherical shape by scanning electron microscopy. Cyclic voltammetry of Cu(NP)-PGE showed an adsorption controlled charge transfer process up to 90.0 mVs−1. The sensor was applied for the determination of glucose using an amperometry technique with a detection limit of [0.44 (±0.01) μM] and concentration sensitivity of [1467.5 (±1.3) μA/mMcm−2]. The preparation of the Cu(NP)-PGE sensor was reproducible (relative standard deviation = 2.10%, n = 10), very simple, fast, and inexpensive, and the Cu(NP)-PGE is suitable to be used as a disposable glucose sensor.
Keywords: copper nanoparticle, glucose sensor, nonenzymatic, pencil graphite
1. Introduction
The diagnosis and monitoring of diabetes was recently highlighted by a report in which it was stated that 20% of the total world population is affected by this chronic disease [1]. One of the proactive measures needed to control diabetes is the determination of blood glucose concentration with the aid of home-monitoring kits or reliable sensors. Therefore, the development of rapid, simple, and reliable methods of monitoring glucose is one of the most important human needs. As such, many studies have involved the use of enzymes such as glucose oxidase which provides great selectivity and sensitivity toward glucose determination. However, this strategy is limited by the poor stability, high cost of enzymes, complicated immobilization procedure, and critical operational conditions due to sensitivity of the enzyme to pH, temperature, humidity, ionic detergents, and toxic chemicals [2]. Therefore, a variety of approaches have been explored to develop nonenzymatic glucose sensors based on the direct oxidation of glucose for practical applications [3]. Because of their extraordinary physicochemical characteristics, nano-materials such as noble metals [4], metal alloys [5,6], metal nanoparticles [7], metal nanoparticle decorated carbon nanotubes [8], and graphene [9] are of great interest for the fabrication of nonenzymatic sensors. However, most of these nonenzymatic sensors have displayed drawbacks such as the high cost of rare metal precursors, low sensitivity, narrow linear range, poor selectivity, and poisoning by adsorbed intermediates. Among the metals, copper attracts more attention due to its low cost, plenty of morphologies, high specific surface area, the possibility of promoting electron transfer reactions at lower overpotentials, and excellent electro-catalytic activity for glucose oxidation without poisoning [10]. It has been speculated that nonenzymatic electro-oxidation of glucose is greatly enhanced with copper compared with other metals as a result of its electrocatalytic effect mediated by Cu(II)/Cu(III) redox couples [11]. Copper electrodes are employed for the direct determination of carbohydrates in amperometric mode in strongly alkaline mediums without any electrode fouling [12,13]. In the past decade, a variety of copper nanomaterials including CuO [10,14], Cu(OH)2 [15], and Cu(NP) incorporated into carbon nanotubes [15] and graphene [2] have been fabricated and applied for nonenzymatic determination of glucose.
Pencil graphite electrodes (PGE) have some advantages such as high electrochemical reactivity, commercial availability, good mechanical rigidity, disposable, low cost, low technology, and easy of modification. In addition, single-use disposable electrodes may overcome the regeneration drawback of the other solid electrodes. It was reported that PGEs offer a renewal surface which is simpler and faster than polishing procedures, common with solid electrodes [16]. In most reports PGEs were used for enzyme-based glucose determination [17]. For example, more recently PGEs have been used for immobilization of glucose oxidase for glucose biosensing [18,19]. A literature survey revealed that there are no studies to date on the use of copper nanoparticle-modified PGEs for nonenzymatic determination of glucose. Therefore, in the present study a PGE was modified by copper nanoparticles simply via a dip-coating method for the first time. The modified electrode was characterized by cyclic voltammetry and influenced parameters were optimized. The modified electrode was applied for high sensitive amperometric determination of glucose.
2. Methods
2.1. Reagents
Acrylic acid, ammonium persulfate, copper nitrate, hydrazine monohydrate, glucose, and other materials used were all analytical grade obtained from Sigma–Aldrich (Chemical Co, St. Louis, US). All solutions were prepared with double distilled water.
2.2. Synthesis of copper nanoparticles
Acrylic acid was added to 30 mL of distilled water and allowed to stir for 10 minutes. Ammonium persulfate initiator was then added to the reaction mixture and stirred until completion of polymerization and polyacrylic acid synthesis. In a typical synthesis of copper nanoparticles, a mixture of Cu(NO3)2 powder (18.7 mg) and polyacrylic acid (1 mL) were completely dissolved in water (20 mL) under sonication at 60°C for 20 minutes. Then, 0.5M NaOH (1 mL) was added dropwise to the solution to adjust the pH. After sonication for 20 minutes at 60°C, hydrazine (0.064 mL) was added to the sonicated solution. The reaction flask was kept in a water bath at 60°C for 30 minutes. The ruby red color of the solution indicated the formation of copper nanoparticles. This solution was added to the dialysis tube and dialysis was performed in distilled water for 24 hours to remove the excess of hydrazine and other impurities.
2.3. Modification of PGE with copper nanoparticles
PGE was pencil lead (Tombow Co., Ltd. Japan) with a diameter of 0.5 mm that was inserted into a Teflon tube exposing 0.5 cm of its tip (A = 0.15 cm2). Electrical contact was made by soldering a metallic wire to the exposed reverse side of the PGE. To complete the modification, the bare PGE was immersed into the copper nanoparticles colloidal solution for about 60 minutes, then removed, and dried under an atmosphere of argon overnight.
2.4. Characterization
Electrochemical measurements were performed on Potentiostat/Galvanostat Autolab30 (from EcoChemie, NL) in a conventional three-electrode glass cell containing modified PGE as working electrode, a Pt rode as auxiliary electrode, and Ag/AgCl (3MKCl) as reference electrode. UV-visible (Vis) absorbance spectra were measured with a PG Instruments T90 Double Beam UV-Vis Spectrophotometer. Powder (PG Co., Ltd. UK) X-ray diffraction data were obtained with a D8-ADVANCE diffractometer (Bruker AXS, Karlsruhe, Germany) using CuKα radiation with a secondary graphite monochromator. The morphologies of the nanoparticles were investigated via scanning electron microscope (SEM; HITACHI 54160, Japan).
3. Results and discussion
3.1. Characterization of Cu(NP)
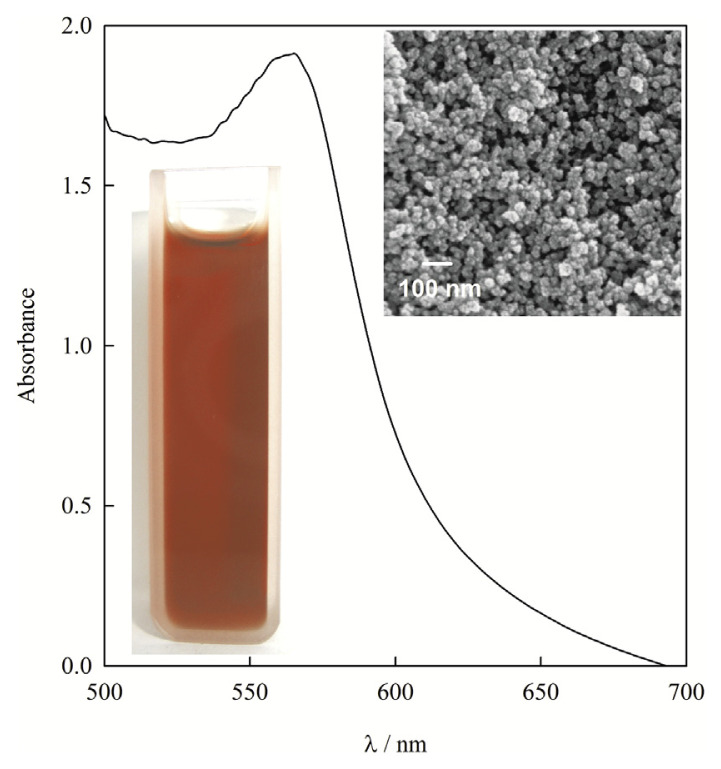
An image of the copper nanoparticles dispersion in distilled water is shown in Figure 1. UV-Vis spectrophotometry was carried out to confirm the formation of copper nanoparticles. A strong characteristic peak around 562 nm was noted for the copper nanoparticles due to the surface plasmon resonance effect [20]. The SEM image of the polyacrylic acid capped copper nanoparticles confirmed the prepared copper nanoparticles were almost homogenously spherical without exhibiting the irregular shapes and aggregation (Figure 1).
Figure 1.
UV–visible spectrum and digital photo of the copper nanoparticles colloidal solution. The inset shows the scanning electron microscopy image of copper nanoparticles.
3.2. Characterization of Cu(NP)-PGE
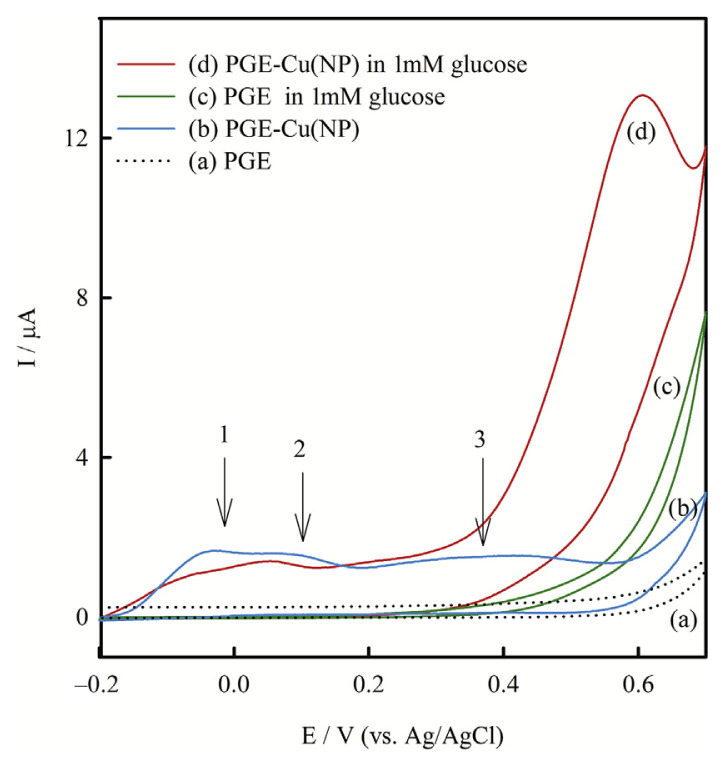
Cu(NP)-PGE was prepared by dipping the PGE into the copper nanoparticles colloidal solution, then removing and drying the modified electrode. Cyclic voltammetry before and after modification was applied to monitor the whole process in preparation of Cu(NP)-PGE. Figure 2 shows the cyclic voltammograms obtained in 0.1M NaOH on the PGE (A) before and (B) after modification by copper nanoparticles. As expected there was no obvious redox peak in the cyclic voltammogram obtained on the bare PGE in the studied potential range. But on the Cu(NP)-PGE cyclic voltammogram revealed three oxidation peaks at potentials about −0.05 V, 0.07 V, and 0.40 V. According to the literature, oxidation features of a copper bulk metal electrode contain three anodic waves at −0.4 V, −0.25 V, and 0.15 V, assigned to the oxidation of Cu to the corresponding oxides of Cu(I), Cu(II), and Cu(III), respectively [13]. Thus, the observed three peaks in this study can be attributed to the oxidation of copper nanoparticles from metallic form to I, II, and III oxidation states. It is worthwhile mentioning that the obtained oxidation peaks potential of copper nanoparticles were significantly more positive than corresponding quantities of copper bulk metal [13]. This behavior can be attributed to the high resistance of copper nanoparticles against oxidation due to the shielding effect of polyacrylic acid around the copper nanoparticles. In brief, the above studies confirmed that copper nanoparticles were successfully immobilized on the PGE.
Figure 2.
Cyclic voltammograms obtained on PGE and PGE-Cu(NP) in the absence (a,b) and in the presence (c,d) of 1 mM glucose, respectively, in 0.1M NaOH at a scan rate of 10.0 mVs−1. NP = nanoparticles; PGE = pencil graphite electrode.
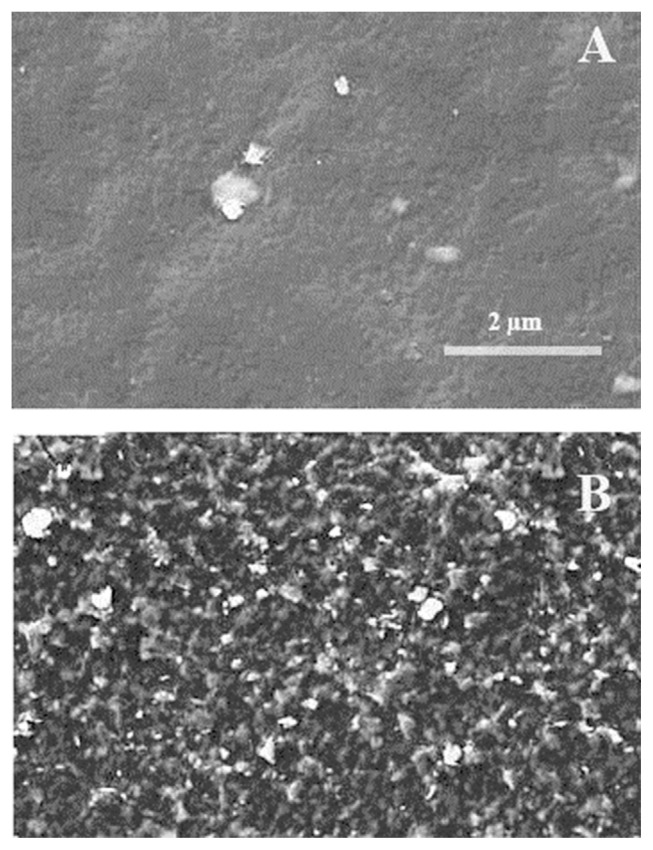
The morphology of the PGE before and after modification with copper nanoparticles was investigated using SEM. As shown in Figure 3A, the PGE surface was almost flat and composed mostly of large unevenness. However, the surface of the modified electrode was much rougher and in addition to the PGE trace homogeneous distribution of individual copper nanoparticles were clearly visible (Figure 3B).
Figure 3.
Scanning electron microscopy image of (A) bare pencil graphite electrode and (B) Cu(nanoparticle)-pencil graphite electrode.
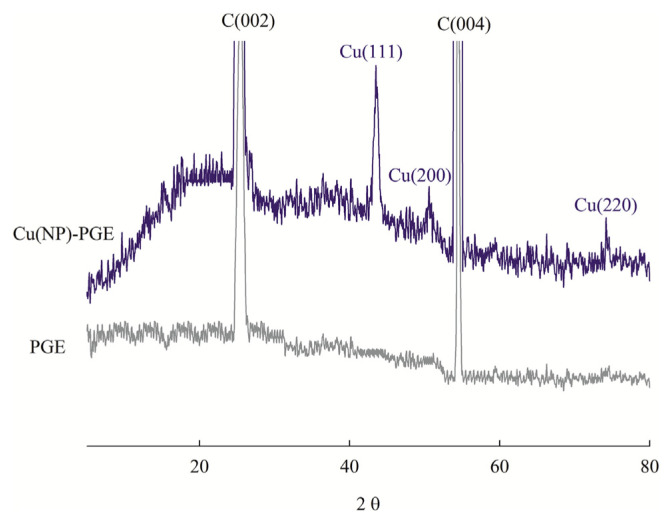
X-ray diffraction patterns of bare PGE and Cu(NP)-PGE were also recorded (Figure 4). Powder X-ray diffraction of bare PGE shows two major peaks at 2θ of 25.5° and 54.5° which corresponded to the graphite (002) and (004) facets, respectively. In the diffraction features of the Cu(NP)-PGE in addition to the PGE peaks, three new peaks at 2θ of 43.2°, 50.6°, and 74.2° were obtained. These peaks were corresponding to the (111), (200), and (220) facets of metallic copper, indicating crystallinity of the particles.
Figure 4.
Powder X-ray diffraction pattern of bare pencil graphite electrode (PGE) and Cu(NP)-PGE.
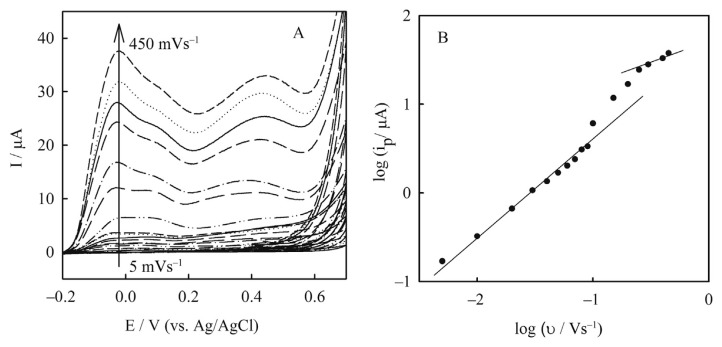
Dependency of the peaks current on scan rate enables distinguishing between diffusion or adsorption controlled charge transfer process. Figure 5A shows the cyclic voltammograms of the Cu(NP)-PGE obtained in 0.1M NaOH at different scan rates. A plot of the logarithm of first oxidation peak currents (log ip) versus the logarithm of scan rates (log υ) is presented in Figure 5B. Two linear ranges were obtained at scan rates from 5–90 mVs−1 and 200–450 mVs−1 as the following equations, respectively:
Figure 5.
(A) Cyclic voltammograms of Cu(nanoparticle)-pencil graphite electrode in 0.1M NaOH at different scan rates from inner to outer: 5.0–450.0 mVs−1; (B) plot of logarithm of first oxidation current of the peaks (log ip) versus logarithm of scan rates (log υ).
| (1) |
| (2) |
The slope obtained by linear regression at scan rates 5–90 mVs−1 was 0.94 corresponding to adsorption controlled charge transfer process over this scan rate range. However, at scan rates 200–450 mVs−1 the slope was 0.52 indicating diffusion controlled charge transfer process at higher scan rates. At scan rates between 90 mVs−1 and 200 mVs−1 the peaks current was controlled by both adsorption and diffusion charge transfer process [23].
3.3. Electrocatalysis of glucose at the Cu(NP)-PGE
Figure 2 shows the cyclic voltammograms in 0.1M NaOH containing 1mM glucose obtained on the (C) bare and (D) Cu(NP) modified PGE. Upon the addition of 1mM glucose, there was a dramatic enhancement of anodic peak currents due to strong electrocatalytic activity of copper nanoparticles for glucose oxidation. The anodic peak potential of oxidation of glucose at Cu(NP)-PGE was about 0.55 V. However, on the bare PGE the weak peak was observed at a more positive potential. Consequently, compared with PGE, the Cu(NP)-PGE had a great reduction of overpotential and dramatic increase of peak currents were achieved in the presence of glucose. It has been proposed that Cu(III) species may act as an electron transfer mediator for the oxidation of glucose as the following equations [12]:
| (3) |
| (4) |
As a result, the above electrochemical studies revealed the strong electrocatalytic activity of Cu(NP)-PGE towards nonenzymatic glucose oxidation. Therefore, the modified electrode can be applied as a sensor for electrochemical determination of glucose.
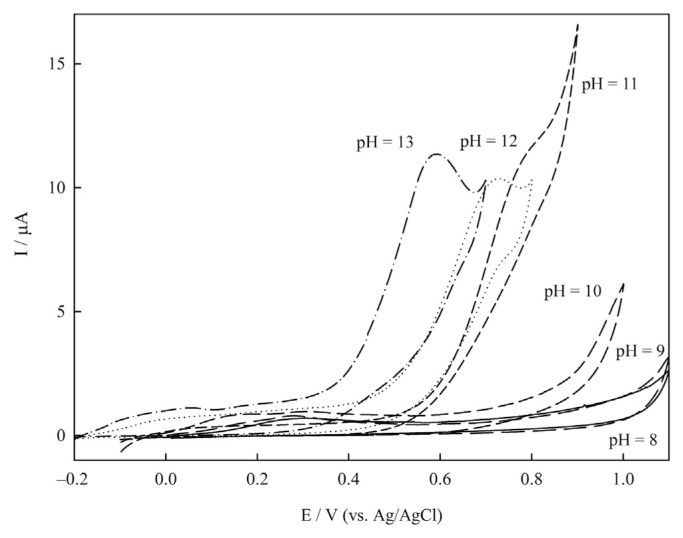
In order to optimize the electrocatalytic activity of the sensor, the effect of pH was investigated. The responses of the Cu(NP)-PGE in 1mM glucose solutions at different pH values (8–13) are presented in Figure 6. As can be seen, at pH < 10 the electrode did not show electrocatalytic activity for glucose oxidation. However, at pH > 10 the peak currents were increased dramatically indicating the electrocatalytic activity of the sensor. By increasing the pH from 10 to 13 the anodic peaks potential shifted to less positive values and the best condition was achieved at pH 13. Therefore, pH 13 was selected as the optimized value for glucose determination on Cu(NP)-PGE.
Figure 6.
Cyclic voltammograms of Cu(nanoparticle)-pencil graphite electrode at scan rate 10.0 mVs−1 in different pHs from 8 to 13.
3.4. Determination of glucose
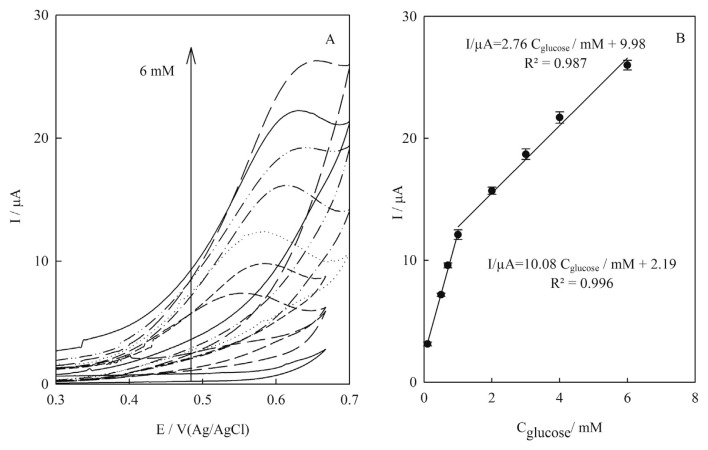
Figure 7A shows the influence of concentration of glucose on the cyclic voltammograms of the Cu(NP)-PGE. Upon the addition of glucose, the anodic peak currents were increased accordingly and the peaks potential shifted to a more positive direction. The shift of the peak potential value of glucose oxidation can be explained by the increase of glucose molecules on the surface of the electrode. In higher concentrations of glucose, the amount of molecules on the surface of the electrode will also be higher. As these molecules are oxidized, their concentration near the electrode falls, which means that in order to maintain surface concentrations, a large flow of species is generated from the middle of the solution to its surface.
Figure 7.
(A) Cyclic voltammograms of Cu(nanoparticle)-pencil graphite electrode in 0.1M NaOH containing glucose in various concentrations from down to top: 0.0–6.0mM at scan rate 10.0 mVs−1; (B) linear correlation between electrocatalytic peak currents of cyclic voltammograms of Cu(nanoparticle)-pencil graphite electrode and glucose concentrations from 0.1mM to 1mM and from 1.0mM to 6.0mM. Error bars indicated standard deviations (n = 3).
Two linear dynamic ranges were obtained from 0.1mM to 1.0mM and from 1.0mM to 6.0mM glucose (Figure 7B). The merits extracted from cyclic voltammograms were poor and cyclic voltammograms became deformed at higher concentrations of glucose. Therefore, due to these disadvantages [21], the merits obtained in this part did not satisfy our requirements for quantitative chemical analysis; i.e., low detection limit, high sensitivity, and high accuracy [22].
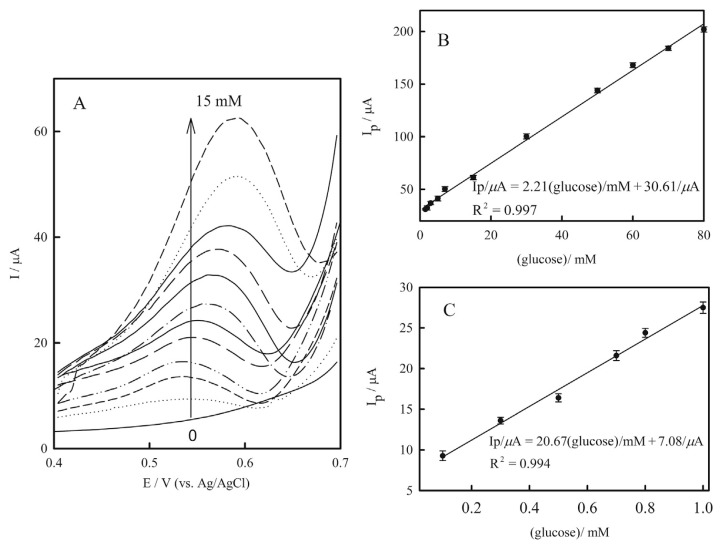
Differential pulse voltammetry, which has the benefits of high sensitivity and a low detection limit (small contribution of background in the analytical signal) was also employed for quantitative determination of glucose. Figure 8A shows the influence of the concentration of glucose on the differential pulse voltammograms of the Cu(NP)-PGE. Upon the addition of glucose, the peaks current was increased. Again, two linear dynamic ranges were observed from 0.1mM to 1.0mM and from 1.5mM to 80.0mM glucose (Figures 8B and 8C). The differential pulse voltammograms became deformed at glucose concentrations more than 15mM.
Figure 8.
(A) Differential pulse voltammograms of Cu(nanoparticle)-pencil graphite electrode in 0.1M NaOH containing glucose in various concentrations from down to top: 0.0–15.0mM, at scan rate 25.0 mVs−1. Linear correlation between electrocatalytic peak currents of differential pulse voltammograms of Cu(nanoparticle)-pencil graphite electrode and glucose concentrations: (B) from 0.0mM to 80.0mM; and (C) from 0.0mM to 0.1mM. Error bars indicated standard deviations (n = 3).
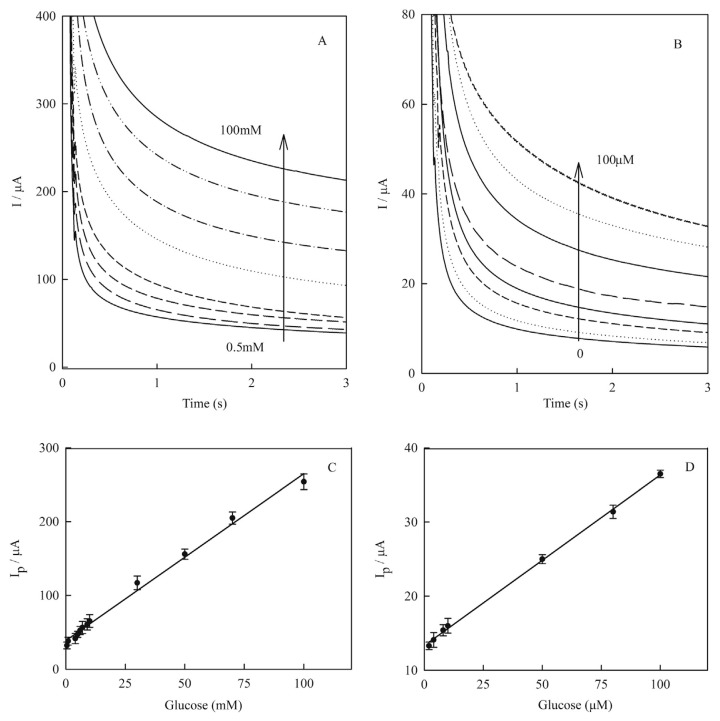
In order to obtain a broader linear dynamic range and lower detection limit, an amperometry technique was employed under the optimized experimental conditions and applied potential of 0.55 V. Figure 9 shows the current–time response of the Cu(NP)-PGE for different concentrations of glucose (A) from 0.5mM to 100.0mM and (B) from 0.0mM to 100.0μM. The amperometric signals showed a linear correlation to glucose concentrations in the range from 0.1mM to 100.0mM and from 1.0μM to 100.0μM glucose as shown in the following equations, respectively (Figures 9C and 9D):
Figure 9.
Amperometric response curve of Cu(nanoparticle)-pencil graphite electrode in 0.1M NaOH containing glucose in various concentrations: (A) from 0.5mM to 100.0mM; (B) from 0.0mM to 100.0μM. Amperometry was carried out at fixed applied potential of +0.55 V. Linear correlation between electrocatalytic peak currents of amperograms of Cu(nanoparticle)-pencil graphite electrode and glucose concentrations: (C) from 0.5mM to 100.0mM as {Ip/μA = 2.26(±0.06) [glucose]/mM + 38.66(±2.45)/μA, R2 = 0.992}; and (D) from 1.0mM to 100.0μM as {Ip/μA = 0.23(±0.003) [glucose]/μM + 13.31(±0.18)/μA, R2 = 0.998}. Error bars indicated standard deviations (n = 3).
| (5) |
| (6) |
An amperogram was obtained on the new electrodes and for each glucose concentration four independent measurements were performed. The standard deviations of each of the four measurements were calculated and were represented as error bars on the calibration curves. The detection limit of glucose was estimated from Eq. (6) as: [0.44 (±0.01) μM] (based on signal-to-noise ratio = 3). From the slope of the calibration curve the concentration sensitivity was obtained as [1467.5 (±1.3) μA/mMcm−2].
There are many papers in the literature dealing with nonenzymatic glucose sensors based on various copper nanostructures [2,8,10,15,23–26]. The determination performance of the sensor fabricated in this work was compared with other recently reported nonenzymatic glucose sensors (Table 1). Comparison of the performance of Cu(NP)-PGE with the others revealed that the obtained figures of merit were consistent or even more better than the values recently reported for glucose determination. Noteworthy, in most cases, our obtained sensitivity was much better than others [2,8,10,15,23–26]. The observed high sensitivity can be attributed to the high specific surface area due to the homogeneous distribution of copper nanoparticles on the PGE. More recently nonenzymatic glucose sensors based on Ni/Cu-nanoparticle modified TiO2 nanotubes was developed, and in spite of the slightly higher sensitivity it has a higher detection limit and more positive detection potential than ours [24,27].
Table 1.
Analytical parameters of glucose nonenzymatic electrochemical biosensors using various copper nanostructures.
| Electrode | Modifier | Detection potential (mV) | Sensitivity μA/mMcm−2 | Detection limit (μM) | Refs |
|---|---|---|---|---|---|
| GCE | Cu(NP)-graphene | 500 | 157.2a | 0.5 | [2] |
| GCE | Cu/PEI/MWCNT | 350 | 714.4a | 0.5 | [8] |
| GCE | CuO nanocubes–graphene | 590 | 1360 | 0.7 | [10] |
| Cu foil | Cu(OH)2 nanotubes | 400 | 418b | 0.5 | [15] |
| GTE | Cu nanowires | 550 | 1100 | 1.6 | [23] |
| GCE | Cu2O/NiOx/graphene oxide | 600 | 285 | 0.4 | [25] |
| GCE | CuO/mesoporous carbon | 450 | 1154.1 | 0.1 | [26] |
| TiO2 | Cu/Ni (NP) | 600 | 1590.9 | 5 | [27] |
| GCE | CuO/graphene | 600 | 1065 | 1 | [24] |
| PGE | Cu(NP) | 550 | 1467.5 | 0.44 | This work |
GCE = glassy carbon electrode; GTE = graphene transparent electrode; MWCNT = multiwalled carbon nanotubes; NP = nanoparticles; Pei = polethylenimine.
Calculated.
μA/mM.
3.5. Interference study
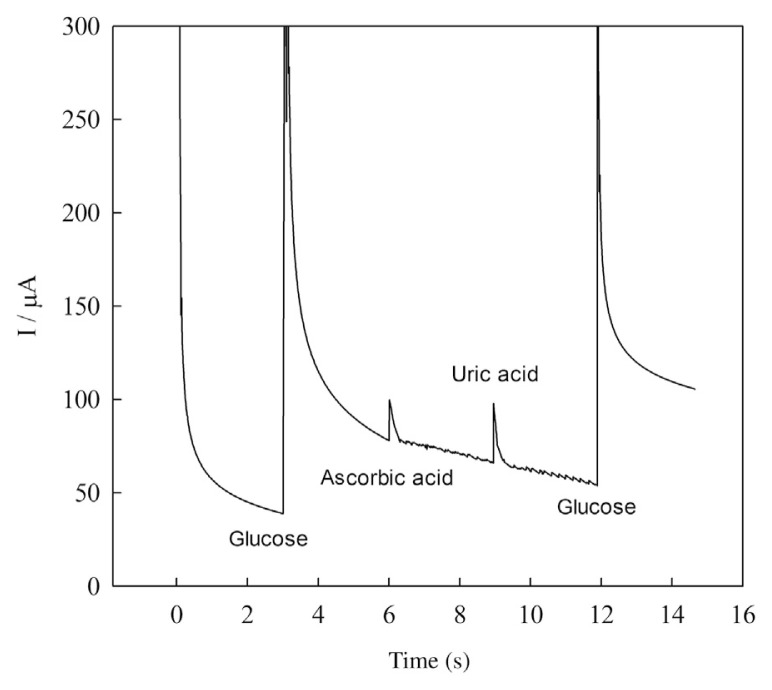
The oxidizable compounds such as ascorbic acid and uric acid normally coexist with glucose in real samples. The normal physiological level of glucose is about 3–8mM, which is much higher than the levels of endogenous ascorbic acid (0.125mM) and uric acid (0.33mM) [24]. Considering the concentration of glucose is at least 10 times that of interferents in human blood serum, the interference test was carried out using 1mM glucose and 0.1mM of interfering species. The amperometric response of the sensor in the presence of 1mM glucose was not affected significantly (< 4%) due to the addition of 0.1mM of ascorbic acid and uric acid (Figure 10). These results indicated that the sensor was selective to glucose even in the presence of interfering species normally found in biological samples. Such selectivity may be attributed to the negatively charged capping agent polyacrylic acid which can electrostatically repel the negatively charged interferents.
Figure 10.
Amperometric response curve of Cu(nanoparticle)-pencil graphite electrode in 0.1M NaOH after successive addition of glucose, ascorbic acid, uric acid, and glucose, respectively.
To evaluate the applicability of the Cu(NP)-PGE in real samples, it was applied to determine glucose in human blood serum samples. In this case, the serum sample was diluted 100 times with the 0.1M NaOH solution, and measured under optimal experimental conditions using the standard addition method. Table 2 displays the determination results from four different serum samples. The results were comparable with that measured by traditional spectrophotometric methods based on glucose oxidase and peroxidase reaction.
Table 2.
Determination of glucose concentration in the human blood serum samples.
| Samples | Cu(NP)-PGE mM ± SD (n = 3) | Spectrophotometry mM ± SD (n = 3) |
|---|---|---|
| 1 | 6.51 ± 0.12 | 6.55 ± 0.07 |
| 2 | 5.43 ± 0.13 | 5.49 ± 0.08 |
| 3 | 7.52 ± 0.14 | 7.43 ± 0.08 |
| 4 | 5.62 ± 0.12 | 5.58 ± 0.06 |
NP = nanoparticles; PGE = pencil graphite electrode; SD = standard deviation.
3.6. Reproducibility and durability
To show the reproducibility of the sensor, the cyclic voltammograms carried out at 100 mVs−1 for 10 independent fabricated sensors. The relative standard deviation (RSD) of the sensors response to 1.0mM glucose was 2.10% indicating high reproducibility. Moreover, the sensors were soaked in 0.1M NaOH, stored under vacuum desiccators, and then tested daily to measure durability. The RSD of the sensors’ response to 1.0mM glucose was 3.91% during 30 days indicating high durability.
The only disadvantage of the Cu(NP)-PGE was its poor repeatability at sequential cycling—at the second cycle only the background current were observed. This behavior probably was due to the dissolution of the oxidized copper ions into the solution. This problem was resolved somewhat by extending the reduction potential toward more negative values. For example, when the reduction potential range was extended from −0.2 V to −0.6 V, the peaks were observable subsequently at sequential scans, although the current of the peaks were decreasing gradually at each cycle. However, single-use disposable electrodes may overcome the regeneration drawback of the solid electrodes. PGEs offer a renewal surface which is simpler and faster than the polishing procedures common with solid electrodes [16]. Therefore, since the method of modification of the PGE by copper nanoparticles was reproducible, very simple, fast, and inexpensive, no attempt was carried out for improvement of the electrode repeatability. Therefore, the Cu(NP)-PGE is meant to be used once and then discarded as a disposable electrode.
A nonenzymatic glucose sensor based on PGEs modified by copper nanoparticles was prepared. The obtained figures of merit were more or less consistent with the values recently reported in the literature (Table 1) for high sensitive determination of glucose. Fabrication of the sensor was reproducible, very simple, fast, and inexpensive, and was meant to be used as disposable glucose sensor. Since the development of rapid, simple, and reliable methods of monitoring glucose is one of the most important human needs, this nonenzymatic glucose sensor will lead us to possible breakthroughs in designing the glucose sensing devices that realize innovative and powerful detection.
Acknowledgments
The authors gratefully acknowledge the Payame Noor University of Miandoab, Tehran, Iran for providing facilities for this work.
Footnotes
Conflicts of interest
The authors have nothing to disclose.
REFERENCES
- 1. Wang HC, Lee AR. Recent developments in blood glucose sensors. J Food Drug Anal. 2015;23:191–200. doi: 10.1016/j.jfda.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luo J, Jiang S, Zhang H, Jiang J, Liu X. A novel nonenzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal Chim Acta. 2012;709:47–53. doi: 10.1016/j.aca.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 3. Park S, Boo H, Chung TD. Electrochemical nonenzymatic glucose sensors. Anal Chim Acta. 2006;556:46–57. doi: 10.1016/j.aca.2005.05.080. [DOI] [PubMed] [Google Scholar]
- 4. Cherevko S, Chung CH. Gold nanowire array electrode for nonenzymatic voltammetric and amperometric glucose detection. Sens Actuators B Chem. 2009;142:216–23. [Google Scholar]
- 5. Ryu J, Kim K, Kim HS, Hahn HT, Lashmore D. Intense pulsed light induced platinum–gold alloy formation on carbon nanotubes for nonenzymatic glucose detection. Biosens Bioelectron. 2010;26:602–7. doi: 10.1016/j.bios.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 6. Cui HF, Ye JS, Zhang WD, Li CM, Luong JHT, Sheu FS. Selective and sensitive electrochemical detection of glucose in neutral solution using platinum–lead alloy nanoparticle/carbon nanotube nanocomposites. Anal Chim Acta. 2007;594:175–83. doi: 10.1016/j.aca.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 7. Inbaraj SB, Chen BH. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J Food Drug Anal. 2016;24:15–28. doi: 10.1016/j.jfda.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu HX, Cao WM, Li Y, Liu G, Wen Y, Yang HF, Yang SP. In situ growth of copper nanoparticles on multiwalled carbon nanotubes and their application as nonenzymatic glucose sensor materials. Electrochim Acta. 2010;55:3734–40. [Google Scholar]
- 9. Wu JW, Wang CH, Wang YC, Chang JK. Ionic-liquid-enhanced glucose sensing ability of non-enzymatic Au/graphene electrodes fabricated using supercritical CO2 fluid. Biosens Bioelectron. 2013;46:30–6. doi: 10.1016/j.bios.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 10. Luo L, Zhu L, Wang Z. Nonenzymatic amperometric determination of glucose by CuO nanocubes–graphene nanocomposite modified electrode. Bioelectrochemistry. 2012;88:156–63. doi: 10.1016/j.bioelechem.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 11. Lu LM, Zhang XB, Shen GL, Yu RQ. Seed-mediated synthesis of copper nanoparticles on carbon nanotubes and their application in nonenzymatic glucose biosensors. Anal Chim Acta. 2012;715:99–104. doi: 10.1016/j.aca.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 12. Luo P, Prabhu SV, Baldwin RP. Constant potential amperometric detection at a copper-based electrode: electrode formation and operation. Anal Chem. 1990;62:752–5. [Google Scholar]
- 13. Yeo IH, Johnson DC. Anodic response of glucose at copper-based alloy electrodes. J Electroanal Chem. 2000;484:157–63. [Google Scholar]
- 14. Batchelor-McAuley C, Du Y, Wildgoose GG, Compton RG. The use of copper(II) oxide nanorod bundles for the nonenzymatic voltammetric sensing of carbohydrates and hydrogen peroxide. Sens Actuators B Chem. 2008;135:230–5. [Google Scholar]
- 15. Zhou S, Feng X, Shi H, Chen J, Zhang F, Song W. Direct growth of vertically aligned arrays of Cu(OH)2 nanotubes for the electrochemical sensing of glucose. Sens Actuators B Chem. 2013;177:445–52. [Google Scholar]
- 16. Dilgin Y, Kızılkaya B, Ertek B, Işık F, Dilgin DG. Electrocatalytic oxidation of sulphide using a pencil graphite electrode modified with hematoxylin. Sens Actuators B Chem. 2012;171–2:223–9. [Google Scholar]
- 17. Zahir MH, Ab Ghani S. Fabrication of directly polymerized 4-vinylpyridine onto a pencil 2B graphite paste electrode for glucose monitoring. Anal Chim Acta. 1997;354:351–8. [Google Scholar]
- 18. Noiphung J, Songjaroen T, Dungchai W, Henry CS, Chailapakul O, Laiwattanapaisal W. Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal Chim Acta. 2013;788:39–45. doi: 10.1016/j.aca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 19. Sun C, Niu Y, Tong F, Mao C, Huang X, Zhao B, Shen J. Preparation of novel electrochemical glucose biosensors for whole blood based on antibiofouling polyurethane-heparin nanoparticles. Electrochim Acta. 2013;97:349–56. [Google Scholar]
- 20. Wang Y, Biradar AV, Wang G, Sharma KK, Duncan CT, Rangan S, Asefa T. Controlled synthesis of water-dispersible faceted crystalline copper nanoparticles and their catalytic properties. Chemistry. 2010;16:10735–43. doi: 10.1002/chem.201000354. [DOI] [PubMed] [Google Scholar]
- 21.Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. New Jersey: Wiley; 2000. [Google Scholar]
- 22. Pourbeyram S. Electrocatalytic determination of H2O2 on the electrode modified by LBL assembly of polyoxometalates via zirconium ion glue. Sens Actuators B Chem. 2014;192:105–10. [Google Scholar]
- 23. Fan Z, Liu B, Liu X, Li Z, Wang H, Yang S, Wang J. A flexible and disposable hybrid electrode based on Cu nanowires modified graphene transparent electrode for nonenzymatic glucose sensor. Electrochim Acta. 2013;109:602–8. [Google Scholar]
- 24. Hsu YW, Hsu TK, Sun CL, Nien YT, Pu NW, Ger MD. Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim Acta. 2012;82:152–7. [Google Scholar]
- 25. Yuan B, Xu C, Liu L, Zhang Q, Ji S, Pi L, Zhang D, Huo Q. Cu2O/NiOx/graphene oxide modified glassy carbon electrode for the enhanced electrochemical oxidation of reduced glutathione and nonenzyme glucose sensor. Electrochim Acta. 2013;104:78–83. [Google Scholar]
- 26. Zhang J, Ding N, Cao J, Wang W, Chen Z. In situ attachment of cupric oxide nanoparticles to mesoporous carbons for sensitive amperometric nonenzymatic sensing of glucose. Sens Actuators B Chem. 2013;178:125–31. [Google Scholar]
- 27. Li X, Yao J, Liu F, He H, Zhou M, Mao N, Xiao P, Zhang Y. Nickel/copper nanoparticles modified TiO2 nanotubes for nonenzymatic glucose biosensors. Sens Actuators B Chem. 2013;181:501–8. [Google Scholar]