Optimization studya.
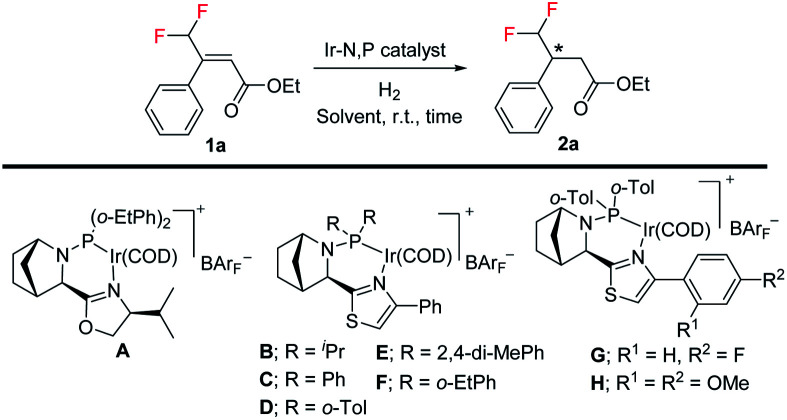
| ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | H2 (bar) | Solvent | Time (h) | Conversion (%) | ee (%) |
| 1 | A (1.0) | 10 | CH2Cl3 | 4 | 95 | 21 |
| 2 | B (1.0) | 10 | CH2Cl2 | 4 | 91 | 91 |
| 3 | C (1.0) | 10 | CH2Cl2 | 4 | 72 | 92 |
| 4 | D (1.0) | 10 | CH2Cl2 | 4 | 99 | 92 |
| 5 | D (0.5) | 5 | CH2Cl2 | 4 | 99 | 92 |
| 6 | D (0.5) | 5 | Toluene | 4 | 99 | 93 |
| 7 | D (0.5) | 5 | PhCF3 | 4 | 99 | 94 |
| 8 | E (0.5) | 5 | PhCF3 | 4 | 99 | 94 |
| 9 | F (0.5) | 5 | PhCF3 | 4 | 99 | 95 |
| 10 | G(0.5) | 5 | PhCF 3 | 4 | 99 | 96 |
| 11 | H(0.5) | 5 | PhCF3 | 4 | 17 | 90 |
Reaction conditions: 0.05 mmol of 1a, 0.5 mL solvent. The conversion was determined by 1H-NMR. Enantiomeric excess was determined by GCMS using a chiral stationary phase.
