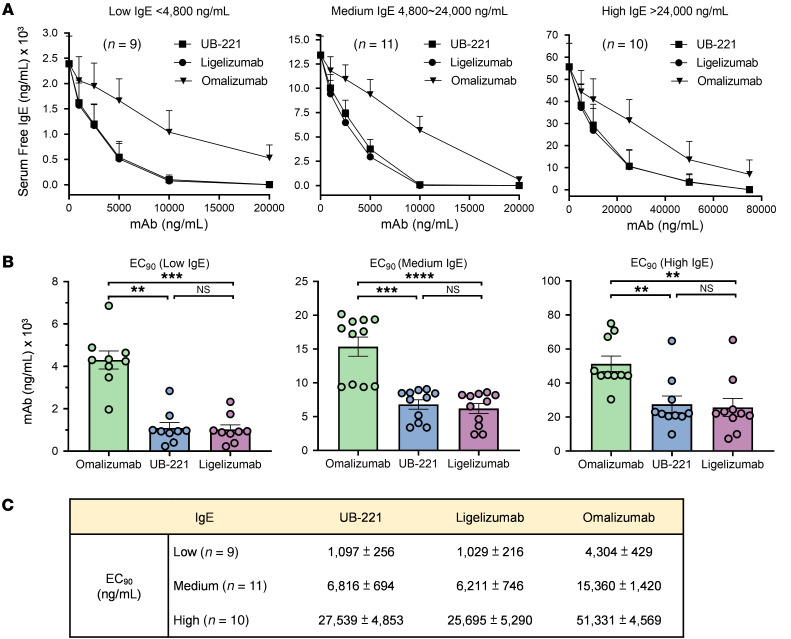
Figure 7. Ex vivo reduction of high-level IgE in sera of patients with atopic dermatitis.
The potency in reducing high IgE in sera from 30 patients with atopic dermatitis was compared for UB-221, ligelizumab (Creative Biolabs, TAB755), and omalizumab, based on a competitive inhibition of IgE binding to FcεRI immobilized on ELISA solid phase. The serum samples were incubated for 1 hour at room temperature with 3 increasing concentrations of anti-IgE mAbs. The remaining free IgE in sera was quantified as described in the Methods. (A) The serum samples collected were grouped into 3 IgE ranges of low (<4,800 ng/mL, n = 9), medium (4,800–24,000 ng/mL, n = 11), and high IgE (>24,000 ng/mL, n = 10). (B) The mAb drug concentrations to achieve a reduction in serum IgE of 90% of the basal IgE levels are illustrated and (C) the EC90 values are presented. All data are shown as mean ± SEM for the low-, medium-, and high-IgE groups. The comparisons were estimated by the Kruskal-Wallis test method. **P < 0.01; ***P < 0.001; ****P < 0.0001. NS, not significant.