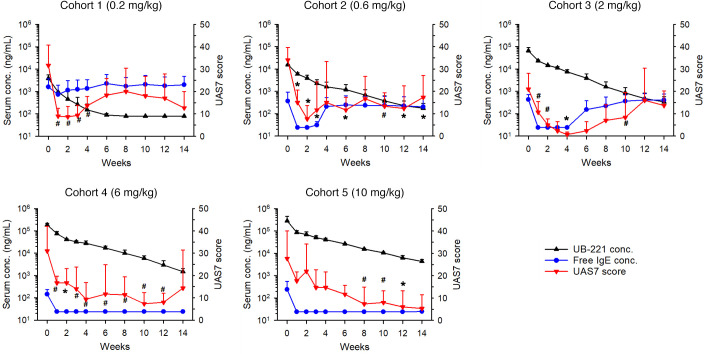
Figure 9. Concurrent serum UB-221 concentration, serum free-IgE level, and UAS7 disease score in patients with CSU after a single i.v. dose of UB-221 in phase I trial.
Shown in parallel are the averaged (mean ± SD) serum concentrations (ng/mL) for both UB-221 (black line) and free IgE (blue line), and CSU symptom relief (reduction) expressed as UAS7 scores (red line) in study participants of 5 dose cohorts (0.2 to 10 mg/kg; n = 3 per cohort) over 14 weeks of the phase I single i.v. UB-221 dose trial. The postdose CSU symptom relief score UAS7 is a combined efficacy marker of hives severity score over 7 days (HSS7) and itch severity score over 7 days (ISS7). The half-life of UB-221 was estimated to be in the range of 16 to 22 days at doses of 0.6 to 10 mg/kg (Supplemental Table 3). The UAS7 changes (red curves) relative to the baseline were estimated and compared by the paired t test for significant differences. Due to the small sample size in each dose cohort that had only 3 participants, P < 0.1 was also assigned to indicate the trend of positive efficacy measured by the decrease in UAS7. #P < 0.1, *P < 0.05.