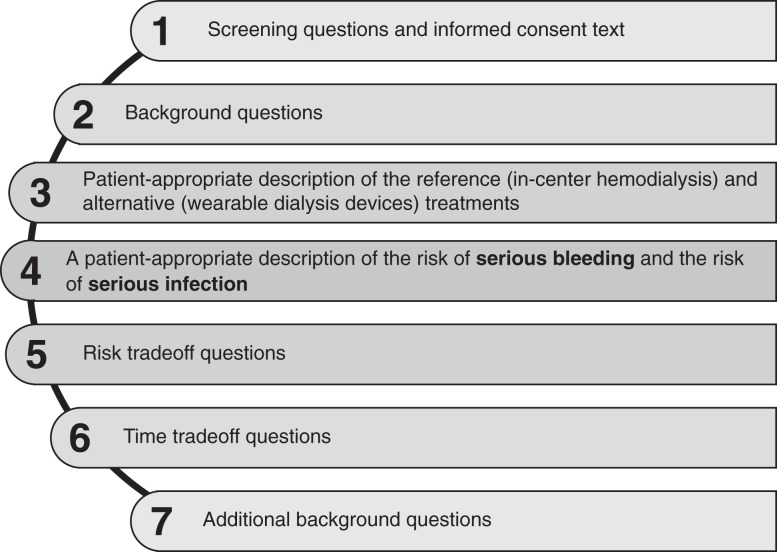
Figure 3.
Survey content overview. The final survey is a 54-item web-based instrument that includes (1) risk trade-off questions designed to quantify the levels of potential risks of serious bleeding and serious infection that patients are willing to accept in exchange for the benefits of the wearable RRT devices; (2) modified time trade-off questions to determine respondents’ discount rate for time until wearable RRT devices are available; (3) comprehension questions to assess understanding of the presented information; and (4) health and background information questions The full survey is available in the Supplemental Material.