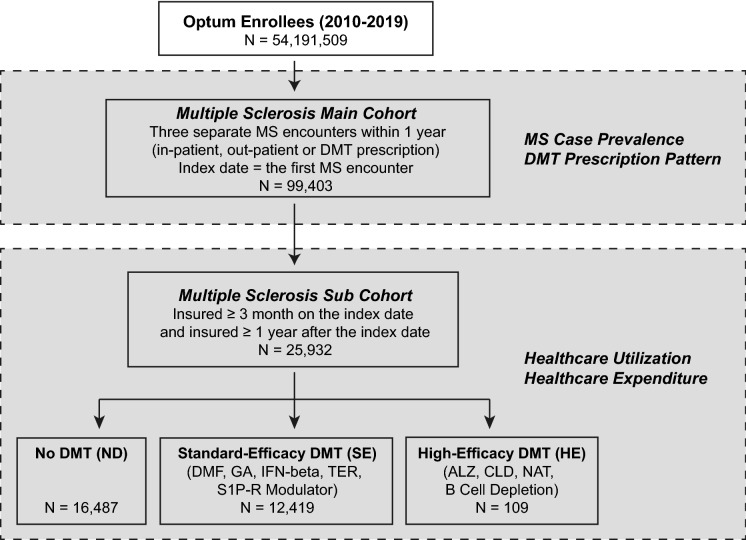
Fig. 1.
Patient selection criteria. DMT disease-modifying therapy, ICD International Classification of Diseases, ALZ alemtuzumab, CLD cladribine, DMF dimethyl fumarate, FGL fingolimod, GA glatiramer acetate, IFN-beta interferon beta (all brands), NAT natalizumab, S1P-R modulator sphingosine-1-phosphate receptor modulator (fingolimod, siponimod), TER teriflunomide. The “No DMT” group includes patients who were not treated with any DMT during the study period. Patients who had been on more than one DMT during the follow-up period belonged to multiple DMT groups, though the claims attributed to a given DMT treatment were counted only after the treatment was started