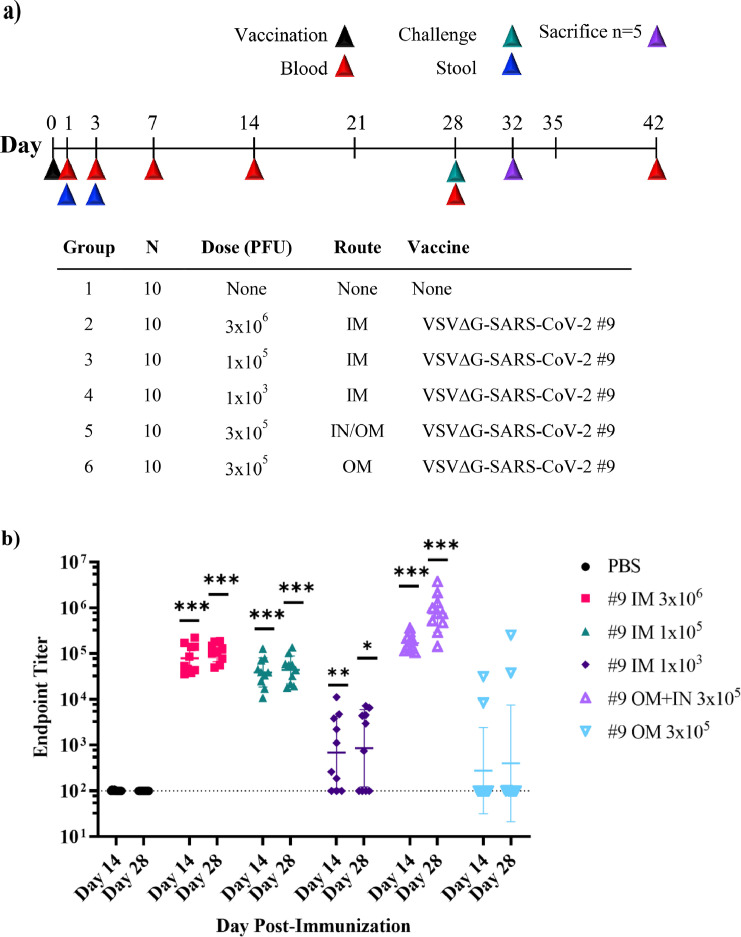
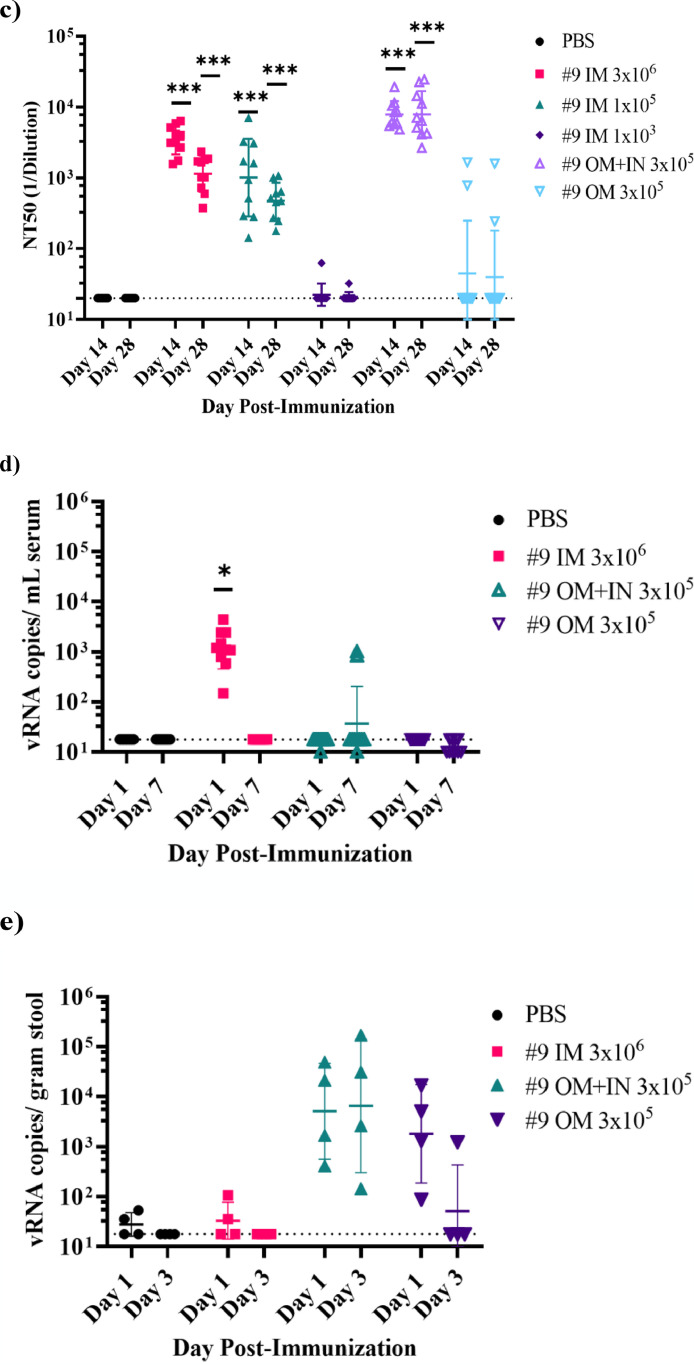
Figure 5.
Immunogenicity of VSV∆G-SARS-CoV-2 #9 in hamsters following intramuscular, intranasal/oral mucosa, or oral mucosa immunisation. The design of the second efficacy study is shown in (a) where six groups of ten hamsters were vaccinated once with VSV∆G-SARS-CoV-2 #9 using the indicated dose and vaccination route. Control animals were unvaccinated. Stool samples were collected from cages on days 1 and 3 following vaccination and blood samples were drawn on days 1, 3, 7, 14, 28, and 42 from all animals. Hamsters were challenged with SARS-CoV-2 on day 28 after which five animals from each group of 10 were euthanised on day 32 (4 days after challenge) to assess SARS-CoV-2 titres in tissues (Figure 6). In (b), sera samples collected on days 14 and 28 were analysed for IgG titres using ELISA plates coated with soluble Spike. The endpoint titres for each animal are graphed and the geometric mean titre and geometric standard deviation (SD) are indicated for each group. (c) Serum neutralising antibodies were quantified by plaque reduction assay using VSV∆G-SARS-CoV-2 #9 and the NT50 titres are graphed for days 14 and 28. The geometric mean titre and geometric standard deviation are indicated for both time points within each group. Neutralising titres (NT50) are defined as the dilution at which there is a 50% reduction in plaques compared with controls. (d) VSV∆G-SARS-CoV-2 RNA copies per millilitre of blood collected on days 1 and 7 after vaccination were quantified by RT-qPCR using primers specific for the VSV matrix gene. Similarly, in (e) VSV∆G-SARS-CoV-2 RNA copies in stool collected from the cages on days 1 and 3 after immunisation were quantified by RT-qPCR. Two hamsters were cohoused per cage thus the stool samples could not be associated with an individual animal. The geometric mean and geometric standard deviation are indicated for both time points within each group. Dashed lines indicate lower limit of assay detection (LOD Endpoint Titre 100, NT50 20, viral RNA stool and serum 17.8 RNA copies). Statistical analysis was performed comparing vaccine cohort responses to control unvaccinated animals (PBS) using an unpaired nonparametric Mann-Whitney test for Endpoint Titre and NT50 (n = 10, *p ≤ 0.05, **p ≤0.005, ***p ≤0.0005) and a two-way ANOVA multiple comparison with Dunnett correction for vRNA copies (n = 4, *p ≤ 0.05). Symbols indicating comparisons to controls that were not significant have been omitted for clarity. Grp, group; IM, intramuscular; IN, intranasal; LOD, limit of detection; OM, oral mucosa; PBS, phosphate-buffered saline; PFU, plaque-forming unit; RT-qPCR, reverse transcriptase quantitative polymerase chain reaction; vRNA, viral ribonucleic acid; VSV, vesicular stomatitis virus.