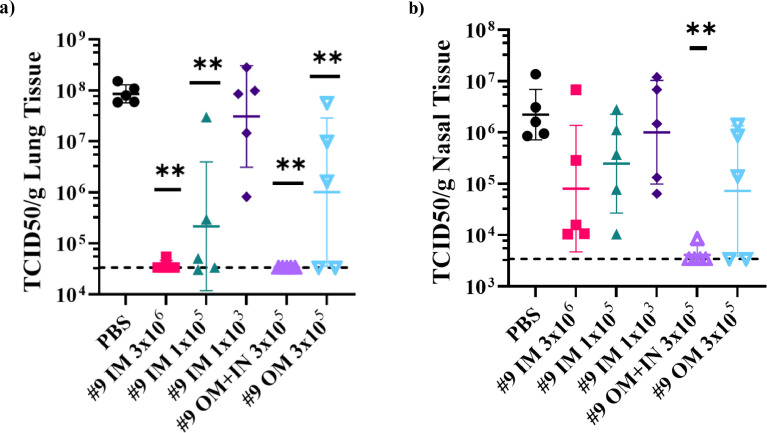
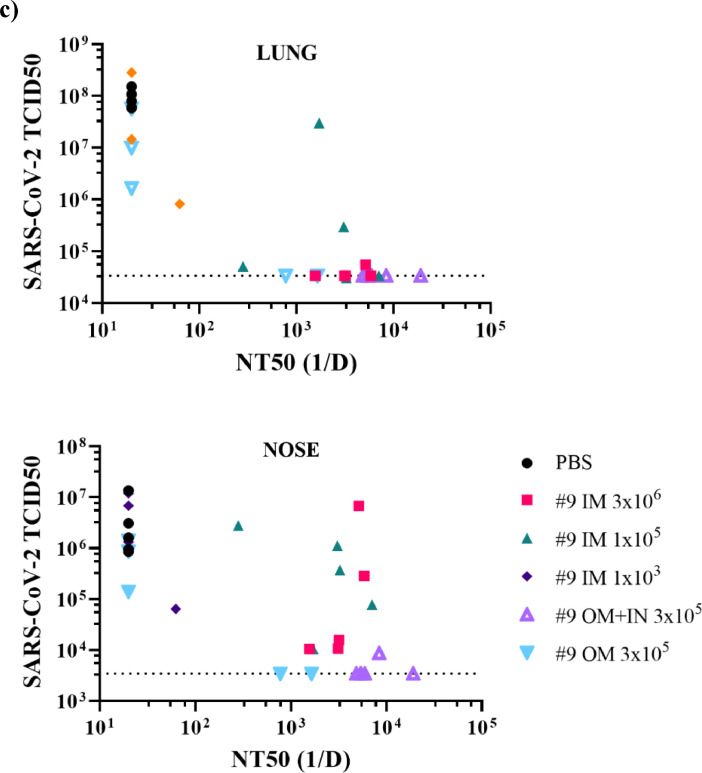
Figure 6.
Protection of hamsters from challenge with SARS-CoV-2 by intramuscular, oral mucosa, or intranasal/oral mucosa immunisation with VSVΔGΔ-SARS-CoV-2. Five of 10 hamsters per group from the second immunogenicity and efficacy study described in Figure 5a were euthanised on day 4 after SARS-CoV-2 challenge. Lung (a) and nasal tissue (b) were processed to quantify infectious SARS-CoV-2 by TCID50 assay and the data are plotted as geometric mean with geometric standard deviation. Statistical analysis was performed comparing vaccine group responses to control unvaccinated animals (PBS) using an unpaired nonparametric Mann-Whitney test (n = 5, **p ≤ 0.005). Symbols indicating comparisons to controls that were not significant have been omitted for clarity. Dashed lines indicate lower limit of assay detection. In (c), SARS-CoV-2 titres in lung and nasal tissues for each animal are graphed (y-axis) against the nAb titre for that animal (x-axis) to illustrate the relationship between nAb titre and effect on SARS-CoV-2 replication. 1/D, 1/dilution; IM, intramuscular; IN, intranasal; OM, oral mucosa; PBS, phosphate-buffered saline; TCID50, tissue culture infectious dose 50; nAb, neutralizing antibody.