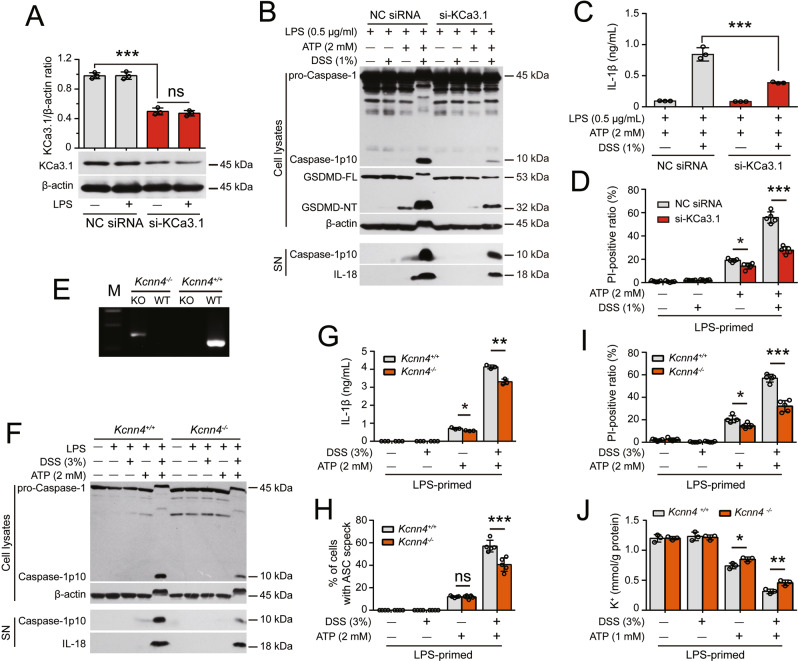
Fig. 6.
Kcnn4 (encoding KCa3.1) knockdown or knockout attenuates the DSS-induced increase in ATP-induced NLRP3 inflammasome activation. After being primed with LPS for 4 h, the cells were treated with DSS for 1 h followed by ATP stimulation. Western blot analysis was performed to examine the levels of the indicated proteins in culture supernatants (SN) and cell lysates. The formation of ASC specks was evaluated by immunofluorescence microscopy, and IL-1β levels in supernatants were measured with a CBA. Lytic cell death was assessed by PI and Hoechst 33342 staining. The K+ concentration in cell lysates was measured by a potassium turbidimetric assay and normalized to the protein concentration in the homogenate (mmol/g protein). Specifically, the expression of KCa3.1 in J774A.1 cells was knocked down for 48 h with a specific siRNA (siRNA-KCa3.1). BMDMs from Kcnn4-/- mice and their littermates were used. A siRNA-KCa3.1 transfection decreased the expression of KCa3.1. The knockdown efficiency of siRNA-KCa3.1 was assessed by Western blotting, and band intensity was quantified with ImageJ software and normalized to β-actin. B–D Knockdown of KCa3.1 expression attenuated the DSS-induce enhancement of ATP-induced inflammasome activation (B), IL-1β release (C), and lytic cell death (D). E KCa3.1-deficient BMDMs were differentiated from Kcnn4−/− mice and genotyped by PCR. Representative electrophoretic results of the PCR analysis of Kcnn4+/+ (320 bp) and Kcnn4−/− (417 bp) BMDMs with specific primers are shown. F–J KCa3.1 depletion attenuated the DSS-induced increase in ATP-induced inflammasome activation (F), IL-1β release (G), ASC speck formation (H), lytic cell death (I), and K+ efflux (J). The data are expressed as the mean ± SD. ns not significant. *p < 0.05, **p < 0.01, ***p < 0.001