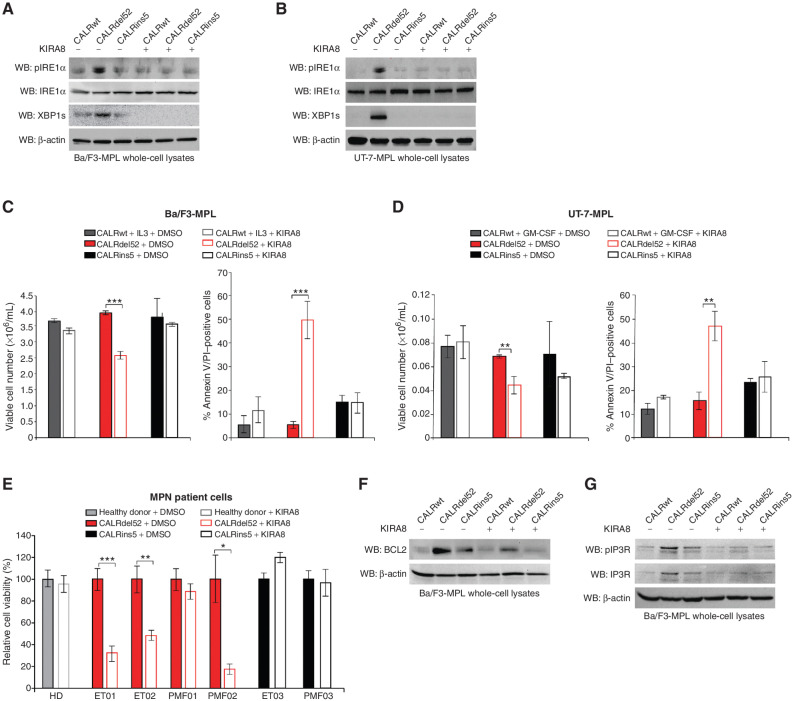
Figure 6.
The IRE1α/XBP1 pathway represents a potential target for therapy in CALRdel52-driven MPNs. A, Western blot analysis for phospho-IRE1α, total IRE1α and XBP1s in Ba/F3-MPL cells expressing calreticulin (CALR) variants treated with or without KIRA8 (5 μmol/L for 4 hours). β-Actin was used as a loading control. B, Western blot analysis for phospho-IRE1α, total IRE1α, and XBP1s in UT-7-MPL cells expressing CALR variants treated with or without KIRA8 (5 μmol/L for 4 hours). β-Actin was used as a loading control. C, Left, total viable cell number at 72 hours post IL3 withdrawal in Ba/F3-MPL cells expressing CALR variants treated with or without KIRA8 (5 μmol/L). Right, quantification of flow cytometric analysis for Annexin V/PI double positivity in Ba/F3-MPL cells expressing CALR variants and treated with or without KIRA8 (5 μmol/L for 48 hours). Each bar represents the average of three independent replicates. Error bars, SD. Significance was determined by two-tailed Student t test (***, P < 0.001). D, Left, total viable cell number at 72 hours post GM-CSF withdrawal in UT-7-MPL cells expressing CALR variants treated with or without KIRA8 (5 μmol/L). Right, quantification of flow cytometric analysis for Annexin V/PI double positivity in UT-7-MPL cells expressing CALR variants and treated with or without KIRA8 (5 μmol/L for 48 hours). Each bar represents the average of three independent replicates. Error bars, SD. Significance was determined by two-tailed Student t test (**, P < 0.01). E, Relative cell viability in peripheral blood mononuclear cells from a healthy donor (HD) or patients with myeloproliferative neoplasms (MPN; patient number depicted below each bar) with CALRdel52 or CALRins5 mutations, treated with or without KIRA8 (500 nmol/L for 48 hours). Viability was determined by CellTiter-Glo assay. Each bar represents the average of three independent replicates. Error bars, SD. Significance was determined by two-tailed Student t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). F, Western blot analysis for BCL-2 in Ba/F3-MPL cells expressing CALR variants treated with or without KIRA8 (5 μmol/L for 24 hours). β-Actin was used as a loading control. G, Western blot analysis for IP3R in Ba/F3-MPL cells expressing CALR variants treated with or without KIRA8 (5 μmol/L for 24 hours). β-Actin was used as a loading control.