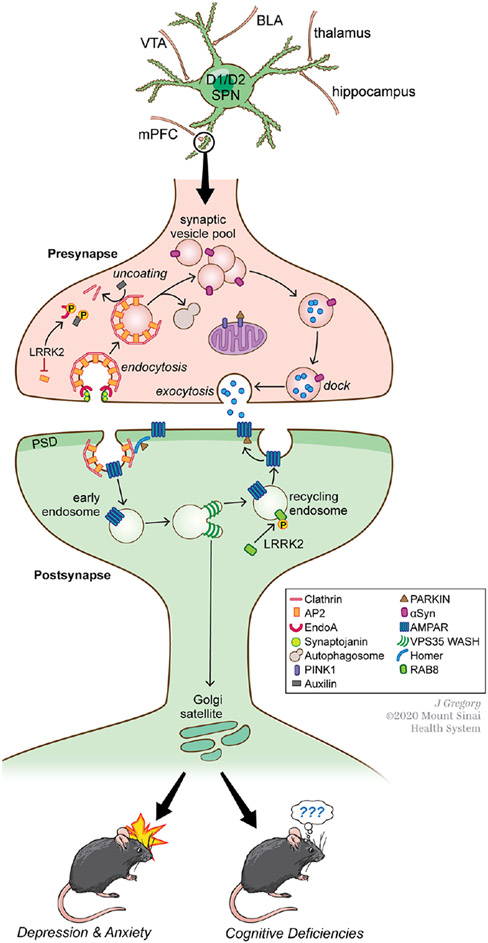
Figure 5.
Several PARK proteins converge functionally on molecular signaling cascades regulating vesicle sorting and trafficking on both pre- and postsynaptic sides of synapses in striatum and elsewhere. (Top) Both D1R (direct)- and D2R (indirect)-spiny striatal projection neurons (SPNs) receive convergent excitatory synaptic input from several sources, as well as dopaminergic input from the substantia nigra or ventral tegmental area (VTA). Through these varied inputs, SPNs mediate a variety of cognitive and motor functions. BLA, basolateral amygdala; mPFC, medial prefrontal cortex. (Middle) A representative example of an excitatory corticostriatal synapse demonstrating the molecular signaling cascades in which various PARK proteins participate. On the presynapse side, LRRK2, α-synuclein (αSyn), Synaptojanin (Synj1), Auxilin, PINK1, and Parkin regulate different aspects of neurotransmitter-containing vesicle exocytocis and endocytocis. On the postsynaptic side, LRRK2, Parkin, and Vps35 regulate vesicle sorting and trafficking, including that of AMPARs important for synaptic transmission and plasticity. PD-associated mutations in these proteins can impair AMPAR trafficking. See text for further details. (Bottom) PD-associated mutations in these PARK proteins in mice affect synaptic neurotransmission and impair plasticity such as LTP at striatal synapses, which in turn can lead to depression- and anxiety-like behaviors and cognitive deficits of the kind that may be relevant to NMS in patients with PD.