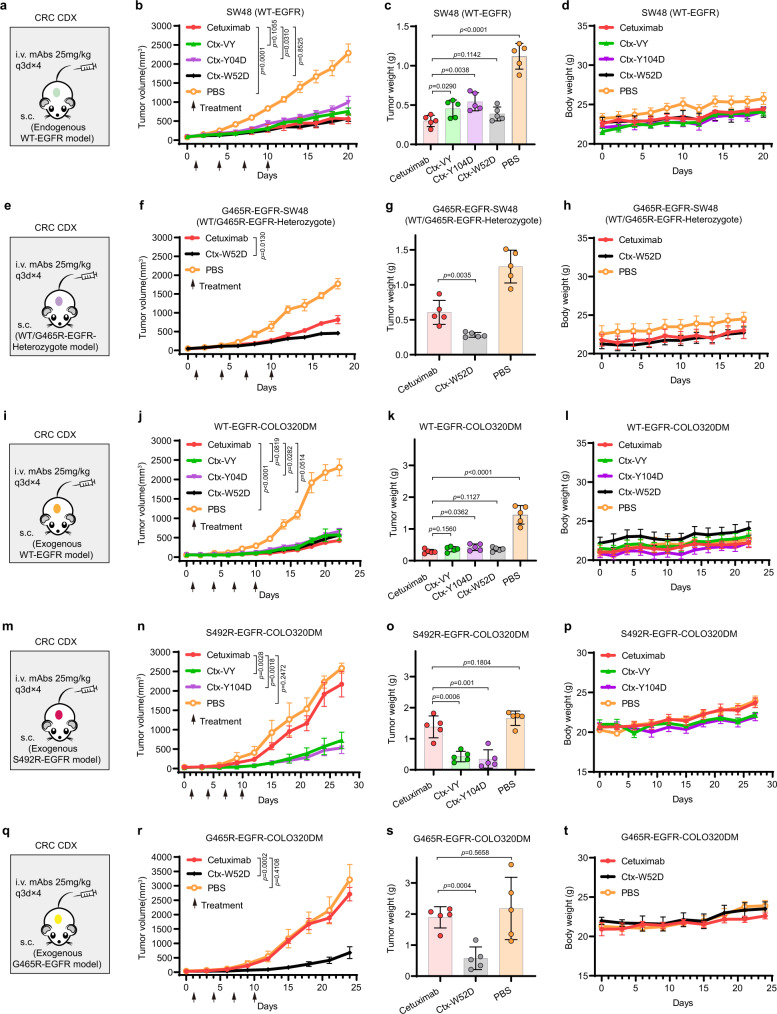
Fig. 7. The in vivo antitumor activities of the cetuximab variants in mouse EGFRWT- or EGFRMut-positive xenograft models.
a The illustration of the SW48 (WT-EGFR) xenograft mouse model establishment, and the administration route and antibody dosages. b The in vivo efficacy of cetuximab and the cetuximab variants in the SW48 xenograft mouse model. c The tumor weight (wet) by the end of study. d The monitoring result of mouse body weight in each experimental or control group. e The G465R-EGFR-SW48 (WT/G465R-EGFR-Heterozygote) xenograft mouse model. f The in vivo efficacy of cetuximab and Ctx-W52D in the G465R-EGFR-SW48 xenograft mouse model. g The tumor weight (wet) by the end of study. h The monitoring result of mouse body weight. i The WT-EGFR-COLO320DM xenograft mouse model. j The in vivo efficacy of cetuximab and its variants in the WT-EGFR-COLO320DM xenograft mouse model. k The tumor weight (wet) by the end of study. l The monitoring result of mouse body weight. m The S492R-EGFR-COLO320DM xenograft mouse model. n The in vivo efficacy of cetuximab, Ctx-VY and Ctx-Y104D in the S492R-EGFR-COLO320DM xenograft mouse model. o The tumor weight (wet) by the end of study. p The monitoring result of mouse body weight. q The G465R-EGFR-COLO320DM xenograft mouse model. r The in vivo efficacy of cetuximab and Ctx-W52D in the G465R-EGFR-COLO320DM xenograft mouse model. s The tumor weight (wet) by the end of study. t The monitoring result of mouse body weight. For all in vivo experiments, the BALB/c nude mice were subcutaneously injected with tumor cells. When the mean tumor volume reached approximately 50 mm3, mice were administered with antibodies at a dosage of 25 mg/kg once every 3 days for four times (q3d×4). All data are shown in mean ± SD values, n = 5 mice for each group (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, N.S. means not significant; two-tailed Student’s t-test). Source data are provided as a Source Data file.