Abstract
BACKGROUND:
A growing body of research has demonstrated that adolescent offspring of depressed parents show diminished responding in the ventral striatum to reward. More recent work has suggested that altered reward responding may emerge earlier than adolescence in offspring at familial risk for depression, although factors associated with neural alterations in childhood remain poorly understood.
METHODS:
We tested whether 6- to 8-year-old children, 49% at heightened risk for depression via maternal history, showed altered neural responding to winning reward. We evaluated whether maternal socialization of positive emotion moderated the association between familial risk and child neural response to reward. Participants were 49 children 6 to 8 years of age (24 with a maternal history of recurrent or chronic depression, 25 with no maternal history of any psychiatric disorder). Children underwent functional magnetic resonance imaging while completing the Doors Guessing Task, a widely used reward guessing task. Mothers reported their use of encouraging and dampening responses to child positive affect.
RESULTS:
Findings demonstrated that children at high familial risk for depression showed lower ventral striatum responding to winning reward relative to low-risk children, but only when mothers used less encouragement or greater dampening responses to their child’s positive emotion expressions.
CONCLUSIONS:
Neural reward alterations in the ventral striatum may emerge earlier than previously thought, as early as 6 to 8 years of age, specifically in the context of maternal discouragement of child positive emotions. Clinical interventions that focus on coaching mothers on how to encourage child positive emotions may be beneficial for supporting child reward-related brain development.
Children with a maternal history of depression have a threefold increased risk for developing depression in their own lifetime (1). Maternal history of chronic or recurrent depression appears to confer the greatest risk for depression in offspring (2). Although risk for depression, as well as for other psychiatric illness, is well established for children with a maternal history of depression, the underlying mechanisms that portend this intergenerational transmission of depression are less understood.
Disruptions in reward-related brain function appear to be one underlying mechanism associated with the development of depression (3). Neural reward processes are important to emotional wellness (4). A wealth of research has demonstrated that reward function is altered in the context of clinical levels of depression in adolescents and adults (5–7). Specifically, depression is associated with blunted responding in the ventral striatum (VS), a key reward region involved in motivation, pleasure, and goal-directed behavior, and heightened activity in the medial prefrontal cortex and anterior cingulate, and these reward-related neural alterations appear to be associated with lower positive affectivity (7).
Adolescent offspring of depressed parents show similar reward-related neural disruptions, even in the absence of their own psychiatric problems. Specifically, multiple studies have now demonstrated that high-risk adolescent offspring show lower responding in the VS and dorsal striatum (8–11). These reward-related neural disruptions also seem to be predictive of the emergence of depressive symptoms during adolescence (12).
Intriguingly, newly emerging evidence suggests that these reward-related disruptions appear to emerge earlier than adolescence in offspring at risk for depression (13–18). In tandem with long-standing evidence that young children at risk for depression show observed behavioral differences in positive affect (PA) and reward (19,20), children as young as 6 to 10 years of age with a maternal history of depression also show lower dorsal striatal response to social reward and lower VS response to monetary reward (17,18). These findings are important, as they suggest that reward disruptions not only emerge in adolescence, a period characterized by heightened reward sensitivity (21) and the onset of depressive symptoms (22), but also may start much earlier in development. Middle childhood is an important developmental period for exploration, play, and burgeoning social interactions; healthy reward function is key to these processes (23) as well as to recovery from stress (4). Thus, early emerging reward-related neural disruptions may limit children’s socioemotional learning and heighten their vulnerability to early-life stressors.
Certain parent socialization behaviors, such as maternal warmth and encouragement, are integral for supporting healthy reward function (24). In the early years, parental socialization of emotion is defined as parent behaviors (e.g., modeling, contingent responding) that either support or discourage child emotion expressions via implicit or explicit means (25,26). Parent emotion socialization behaviors that support child positive emotion expression often comprise three components: acknowledgement, imitation, and elaboration of child positive emotion expression (27–29). These components serve to encourage and reinforce greater frequency and intensity of child positive emotion expression. In contrast, parental emotion socialization behaviors that dampen child positive emotion expression often include dismissive, invalidating, or punitive responses that can hinder the frequency and intensity of child positive emotions (26,30).
Maternal depression may interfere with the use of positive emotion socialization behaviors (29). This can be problematic, as parental emotion socialization behaviors appear to be related to neural response to reward in the long term (24). For example, low levels of maternal warmth during early childhood and adolescence have been associated with reward-related neural disruptions at age 20, particularly for youths with a maternal history of depression (31). In contrast, maternal positive parenting was shown to buffer the effects of parental depression on reward processing in a sample of 9-year-old children (32). Combined, these findings illustrate that maternal socialization of positive emotion plays an integral role in the development of neural reward systems, particularly for youths who are vulnerable to depression. What is still needed is an understanding of how maternal positive emotion socialization is related to child neural reward systems during childhood. Understanding how maternal emotional socialization behaviors and familial risk for depression work together in tandem to promote child reward function is key to identifying effective interventions to prevent the intergenerational transmission of depression.
The current study evaluated reward-related neural function in children at high and low risk for depression and examined how maternal positive emotion socialization may be associated with reward-related neural disruptions in high-risk children. We hypothesized that high-risk children would show lower responding in multiple reward-related neural regions especially in the VS. We hypothesized that mothers with a history of depression would show lower levels of maternal encouragement and higher levels of maternal dampening in response to child positive emotions relative to mothers without this history. Last, we predicted that maternal socialization of positive emotions (i.e., low encouragement and high dampening) would moderate the association between maternal depression history and child reward-related neural disruptions.
METHODS AND MATERIALS
Participants were 6- to 8-year-old children (N = 49, mean = 6.88 years, SD = 0.75 years) with no lifetime history of psychiatric illness. Participants were 53% female and identified as Black/African American (14%), multiracial (12%), and White (74%). One child identified as Hispanic/Latinx. Mothers reported a mean annual household income of $89,050 (SD = $64,889; range, $0–$300,000). Participants were recruited from existing studies evaluating maternal depression as well as from community advertisements. Children were excluded from the study if they met criteria for any psychiatric disorder (e.g., attention-deficit/hyperactivity disorder, anxiety, depression), developmental disability, or neurological disorder. Children were categorized as high risk for depression (n = 24) if their mothers met criteria for two or more lifetime episodes of major depressive disorder or for dysthymia on the Structural Clinical Interview for DSM-IV (SCID-IV) (i.e., recurrent and/or chronic depression). Mothers were ineligible if they met lifetime criteria for a manic or hypomanic episode, psychotic symptoms, and/or substance dependence. In the high-risk group, 7 mothers were currently depressed at the time of the study. Also, 17 mothers in the high-risk group met lifetime criteria for an anxiety disorder (n = 9 panic disorder, n = 8 social phobia, n = 6 posttraumatic stress disorder, n = 4 generalized anxiety disorder, n = 1 obsessive-compulsive disorder). Children were categorized as low risk for depression (n = 25) if mothers had no lifetime history of any psychiatric disorder.
Participants completed two laboratory visits for the study. At the first visit, mothers and children completed clinical interviews to assess psychiatric history and mothers completed questionnaires about maternal response to child affect and about child affective symptoms. At the second visit, children completed a reward guessing game during a functional magnetic resonance imaging (fMRI) scan. The two visits were on average 31.14 days apart (SD = 19.93 days; range, 1–105 days). The University of Pittsburgh Human Research Protections Office approved all research procedures, and written informed consent was obtained from each child participant and his/her mother.
Measures
Clinical Interviews.
Mothers were interviewed using the SCID-IV to assess maternal current and lifetime history of depression and other psychiatric history. Children and mothers were interviewed separately using the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (K-SADS-PL) by the same interviewer to assess child current and lifetime psychiatric history. Summary scores on the K-SADS-PL were derived from a synthesis of parent and child report. Once again, none of the children in either group met lifetime criteria for any disorder on the K-SADS-PL. For both interviews (SCID-IV and K-SADS-PL), clinical interviewers were trained to reliability by a licensed clinical psychologist. Eighteen percent of the SCID-IV clinical interviews and the K-SADS-PL clinical interviews (n = 12) were double coded by a licensed clinical psychologist, and interrater reliability was high (97.7% diagnostic agreement for SCID-IV; 98.0% for K-SADS).
Maternal Response to Child Affect.
Mothers completed the Parents’ Reaction to Children’s Positive Emotions Scale (33). This scale has demonstrated strong convergent validity with other measures of parental emotion socialization (e.g., coaching and savoring of positive emotions) (34,35) and predictive validity of child outcomes (e.g., depressive symptoms and disruptive behavior problems) (30,36) in prior research. For this scale, mothers rate the likelihood of their response to a series of 12 vignettes in which children typically show a range of positive emotions (e.g., happiness, excitement, curiosity) on a 7-point Likert scale (1 = very unlikely, 7 = very likely). A sample vignette includes the item, “If we are at a wedding and my child is giggling with a cousin seated next to him/her, I would.…” Responses are categorized into 4 subscales: encouragement, explanation, discomfort, and reprimand. Sample responses for this vignette included, “smile and let him/her have fun with his/her cousin” (encouragement), “tell my child that his giggling is disturbing the other people and would redirect his/her attention to some part of the ongoing ceremony” (explanation), “be embarrassed by my child’s behavior” (discomfort), and “frown at my child and firmly tell him/her to be quiet” (reprimand). The encouragement subscale captures mothers’ validation and encouragement of positive emotion expression. The explanation subscale captures explanation of the appropriateness of positive emotion expression in context. The reprimand subscale captures scolding or punishing the child’s positive emotion expression. The discomfort subscale captures disapproval or irritation with the child’s positive emotion expression. Similar to prior research (36), we chose to combine the discomfort and reprimand scales into a maternal dampening composite, as these two scales were highly correlated (r = 0.68) and reflected harsh responses that may dampen child positive emotion expression. We used the dampening composite (α = 0.90) and the encouragement scale (α = 0.69) in separate statistical models for the current study.
Child Affective Symptoms.
Mothers reported on child depressive and anxiety symptoms using the Mood and Feelings Questionnaire (MFQ) (37) and the Screen for Child Anxiety Related Disorders (SCARED) (38), respectively. The MFQ has 34 items, and mothers rated their children’s depressive symptoms within the past 2 weeks on a 3-point Likert scale (0 = not true, 1 = sometimes, 2 = true). The SCARED has 41 items, and mothers rated their children’s anxiety symptoms within the past week on a 3-point Likert scale (0 = not true/hardly ever true, 1 = somewhat true or sometimes true, 2 = very true or often true). Both the MFQ and the SCARED had good internal consistency (α = 0.72 for MFQ and 0.85 for SCARED).
Doors Guessing Task.
We employed a modified version of a widely used reward guessing game, the Doors Guessing Task (13,14,39). In this 6.5-minute, event-related task, participants were presented with an image of two doors and were asked to guess by button press which door was hiding a token. If children guessed correctly, they won that token (win trial). If they guessed incorrectly, they did not win the token for that trial (no win/neutral trial). Children were told to guess quickly or else the computer would guess for them. Behavioral performance was recorded to ensure that children responded with button press within 4 seconds of stimulus presentation for each trial.
Prior versions of this task have utilized monetary incentives and included loss trials as well (e.g., winning 50 cents, losing 25 cents); however, we chose to simplify the task to remove loss trials and use token incentives (13) owing to normative developmental concerns of our sample (i.e., varying understanding of money). The task consisted of 40 trials (20 win, 20 no win/neutral), with a jittered intertrial interval of 1.5 to 9 seconds (see Figure S1). Prior fMRI and event-related potential work has demonstrated that the Doors Guessing Task yields an internally consistent measure of neural reward response with as few as 14 trials per condition (40,41). Children were told before the fMRI scan that they would be able to exchange their tokens for a prize and that the more tokens they won, the better the prize. Unbeknownst to the child, the trials were fixed such that each child won the same trials and the same number of tokens. Children were debriefed following the scan using age-appropriate language. To evaluate neural response to winning reward, we used the contrast of win > no win.
fMRI Acquisition and Preprocessing
Each participant was scanned using a Siemens 3T TIM Trio scanner. Structural images were acquired using magnetization prepared rapid acquisition gradient-echo 192 axial slices, 1.0 mm thick (repetition time/echo time = 2200/3.35 ms, field of view = 256 mm, matrix 256 × 240, flip angle = 9°). Blood oxygen level–dependent functional images for the Doors Guessing Task were acquired in a single run, with a gradient echo-planar imaging sequence and covered 39 axial slices, 3.1 mm thick, beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (repetition time/echo time = 2010/28 ms, field of view = 205 mm, matrix = 64 × 64, flip angle = 90°). All scanning parameters were selected to optimize the quality of the blood oxygen level–dependent signal while maintaining enough slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired and inspected a reference echo-planar imaging scan to confirm the absence of artifacts and good signal across the entire volume of acquisition.
Preprocessing of fMRI data was completed using SPM8 and analyses were conducted in SPM12 (http://www.fil.ion.ucl.ac.uk/spm). Structural images of each participant were segmented to focus on gray matter. For each functional scan, data were realigned to the first volume to correct for head motion and unwarped to correct for static inhomogeneity interactions. Realigned and unwarped images were then coregistered with the participant’s anatomical image. The anatomical image was then spatially normalized into standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model and smoothed with a 6-mm full width at half maximum Gaussian filter. Voxels were resampled during preprocessing to 2 mm3. Preprocessed data were analyzed using first-level random-effects models that account for scan-to-scan variability. Second-level random-effects models that account for participant-to-participant variability were then conducted to determine task-specific regional responses.
Data Analytic Strategy
First, within-sample t tests that included child age and sex were conducted in SPM12. Whole-brain analyses were used to identify significant clusters of interest for the win > no win condition of the task. Subsequently, we evaluated our a priori region of interest (VS) using an anatomically defined mask for the win > no win condition. For both whole-brain and region-of-interest analyses, we used a cluster-forming threshold of puncorrected < .001 and corrected for multiple comparisons using familywise error (FWE) of pFWE < .05 at the peak level (42). Finally, we extracted eigenvariate values from the VS cluster in SPM12 for two regression models in SPSS to 1) evaluate the role of risk status, maternal encouragement, and their multiplicative interaction (risk × maternal encouragement) on VS activity; and 2) evaluate the role of risk status, maternal dampening, and their multiplicative interaction (risk × maternal dampening) on VS activity. Because maternal dampening and maternal encouragement were strongly correlated with one another, they were evaluated in separate models. Simple slope analyses were used to test significant interactive effects, in which we estimated the association of risk status on neural response to winning reward at differing levels of maternal socialization (±1 SD).
RESULTS
Descriptive Statistics
Children at high familial risk for depression did not differ on age, sex, race, family income, or child depressive or anxiety symptoms from children at low risk for depression. Neither maternal encouragement nor maternal dampening of child PA was significantly related to maternal history of depression (see Table 1).
Table 1.
Descriptive Statistics by Group
| Characteristic | High Risk (n = 24) | Low Risk (n = 25) | F48 or χ248, p |
|---|---|---|---|
| Age, Years, Mean (SD) | 6.92 (0.78) | 6.84 (0.75) | F = 0.12, p = 73. |
| Sex, Female, n (%) | 12 (50%) | 14 (56%) | χ2 = 0.18, p = .45 |
| Race, % | 8% Black, 17% multiracial, 75% White | 20% Black, 8% multiracial, 72% White | χ2 = 1.93, p = .38 |
| Family Income, $, Mean (SD) | $77,363.64 ($61,506.52) | $103,333.33 ($67,778.19) | F = 1.61, p = .21 |
| Child Anxiety Symptoms, Mean (SD) | 8.21 (6.81) | 7.12 (5.50) | F = 0.38, p = .54 |
| Child Depressive Symptoms, Mean (SD) | 4.25 (3.29) | 2.64 (3.00) | F = 3.21, p = .08 |
| Maternal Education Level | 16% high school education or lower | 16% high school education or lower | χ2 = 0.00, p = 1.00 |
| Maternal Encouragement of Child PA, Mean (SD) | 53.29 (9.13) | 50.68 (8.61) | F = 1.06, p = .31 |
| Maternal Dampening of Child PA, Mean (SD) | 91.75 (19.84) | 83.42 (24.94) | F = 1.67, p = .21 |
| Ventral Striatal Activity, Mean (SD) | 1.14 (3.30) | 2.75 (3.39) | F = 3.05, p = .09 |
PA, positive affect.
Intercorrelations
Older children had lower VS response to winning reward. As expected, maternal encouragement and maternal dampening of child PA were negatively correlated with one another. Child anxiety symptoms and child depressive symptoms were positively correlated. Greater maternal dampening of child PA was significantly associated with higher child anxiety symptoms on the SCARED (see Table 2).
Table 2.
Intercorrelations of Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 Child Age | – | – | – | – | – |
| 2 Child VS Response | −0.37a | – | – | – | – |
| 3 Child Anxiety Symptoms | −0.24 | 0.06 | – | – | – |
| 4 Child Depressive Symptoms | −0.21 | 0.13 | 0.36b | – | – |
| 5 Maternal Encouragement of Child PA | −0.04 | −0.06 | 0.18 | 0.12 | – |
| 6 Maternal Dampening of Child PA | −0.26 | 0.04 | 0.33b | 0.26 | −0.32b |
PA, positive affect; VS, ventral striatal.
p < .01.
p < .05.
Task Effects
Whole-brain analyses used to examine task effects revealed that winning reward (relative to not winning reward) was associated with greater activation in multiple regions, including the dorsal anterior cingulate, temporoparietal junction, anterior insula, superior parietal lobe, and bilateral occipital lobe (see Table 3). Further, region-of-interest analysis showed that winning reward was associated with activation in the VS (48 voxels, [2, 18, −4], t47 = 4.10, pFWE = .037) (see Figure 1).1 Split-half analyses revealed a significant positive correlation of VS activation to winning reward for even and odd trials (Pearson’s r = 0.29, Spearman-Brown = 0.45, p < .05) (40).
Table 3.
Within-Sample Task Effects for Win Versus No Win
| Region | Voxels | Peak Coordinates | t 47 | pFWE (Peak Level) |
|---|---|---|---|---|
| Temporoparietal Junction, Posterior Insula, Left | 934 | −46, −22, 22 | 6.22 | .004 |
| Occipital Lobe, Left | 1173 | −12, −92, −14 | 6.20 | .004 |
| Occipital Lobe, center | 1091 | 30, −84, −16 | 6.15 | .005 |
| Superior Parietal Lobe, Left | 1000 | −44, −20, 54 | 5.85 | .011 |
| Anterior Insula, Rolandic Operculum, Left | 300 | −48, 4, 2 | 5.46 | .035 |
| Dorsal Anterior Cingulate, BA 32, Left | 472 | −6, 10, 42 | 5.40 | .041 |
Findings are significant using whole-brain analyses at a cluster-forming threshold of p < .001 and using FWE at p < .05 at peak level.
BA, Brodmann area; FWE, familywise error.
Figure 1.
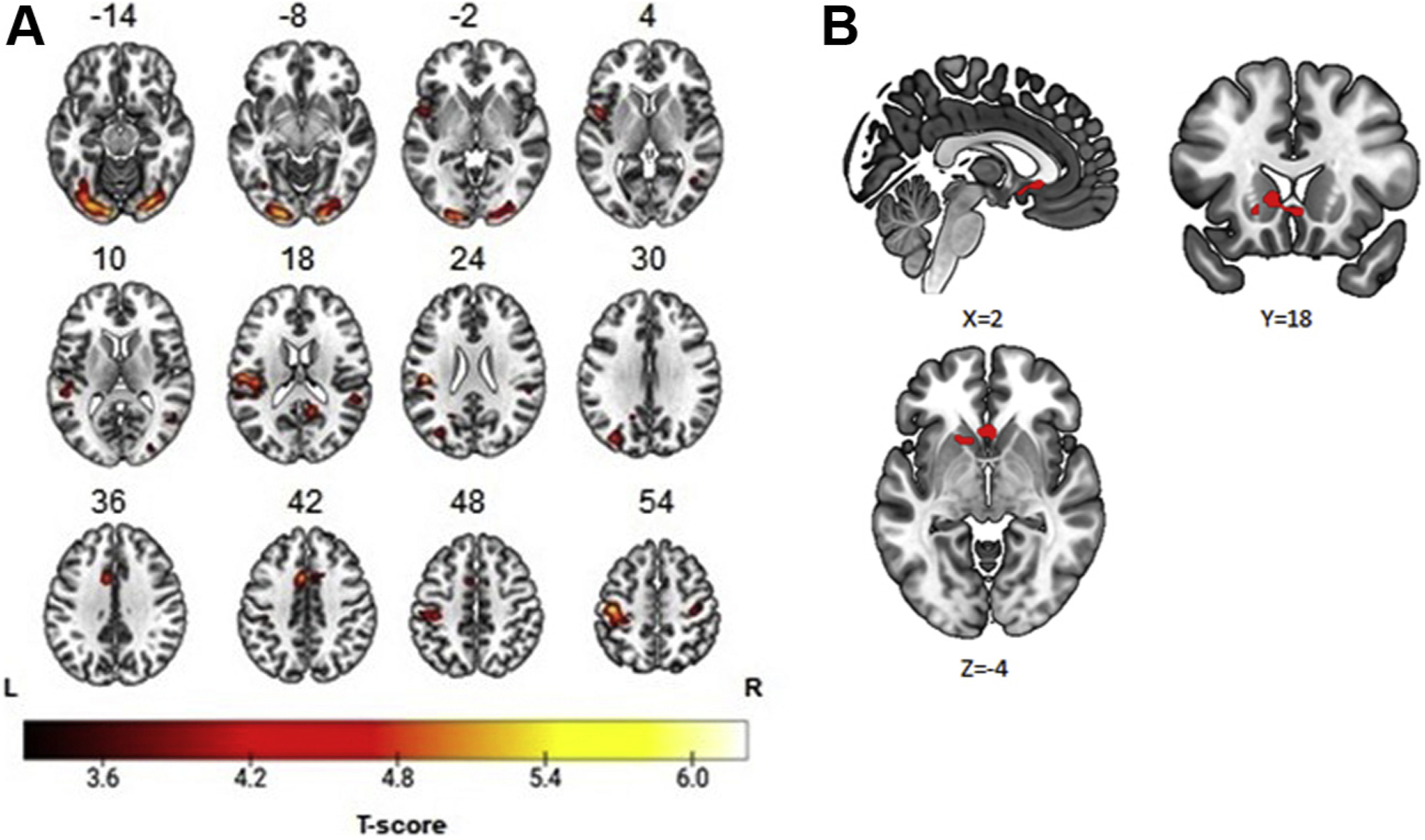
Neural response to winning reward in 6- to 8-year-old children. (A) Whole-brain findings: temporoparietal junction, 934 voxels [−46, −22, 22]; superior parietal lobe, 1000 voxels [−44, −20, −54]; anterior insula, 300 voxels [−48, 4, 2]; dorsal anterior cingulate, 472 voxels [−6, 10, 42]; occipital lobe, left, 1173 voxels [−12, −92, −14]; occipital lobe, right, 1091 voxels [30, −84, −16]. (B) Region-of-interest findings: ventral striatum, 48 voxels [2, 18, −4].
Maternal Encouragement of Child PA and Child VS Response.
Our first regression model revealed that risk status and maternal encouragement of child PA interacted to predict VS activity (β = 0.54, t = 2.11, p = .040) (Table 4). More specifically, analysis of simple slopes revealed that high-risk children had lower VS response to reward compared with low-risk children only when maternal encouragement of child PA was low (β = −0.54, t = −2.73, p = .009). When maternal encouragement was high, there was no significant differences between risk groups on VS activity (β = 0.05, t = 0.27, p = .79) (Figure 2).2
Table 4.
Role of Risk Status, Maternal Encouragement of Child PA, and Their Interactive Effect on Child Ventral Striatal Response to Winning Reward
| Variable | Standardized β | t 48 | p Value |
|---|---|---|---|
| Risk | −0.54 | −2.73 | .009 |
| Maternal Encouragement of Child PA | −0.32 | −1.62 | .113 |
| Risk × Maternal Encouragement of Child PA | 0.54 | 2.11 | .040 |
PA, positive affect.
Figure 2.
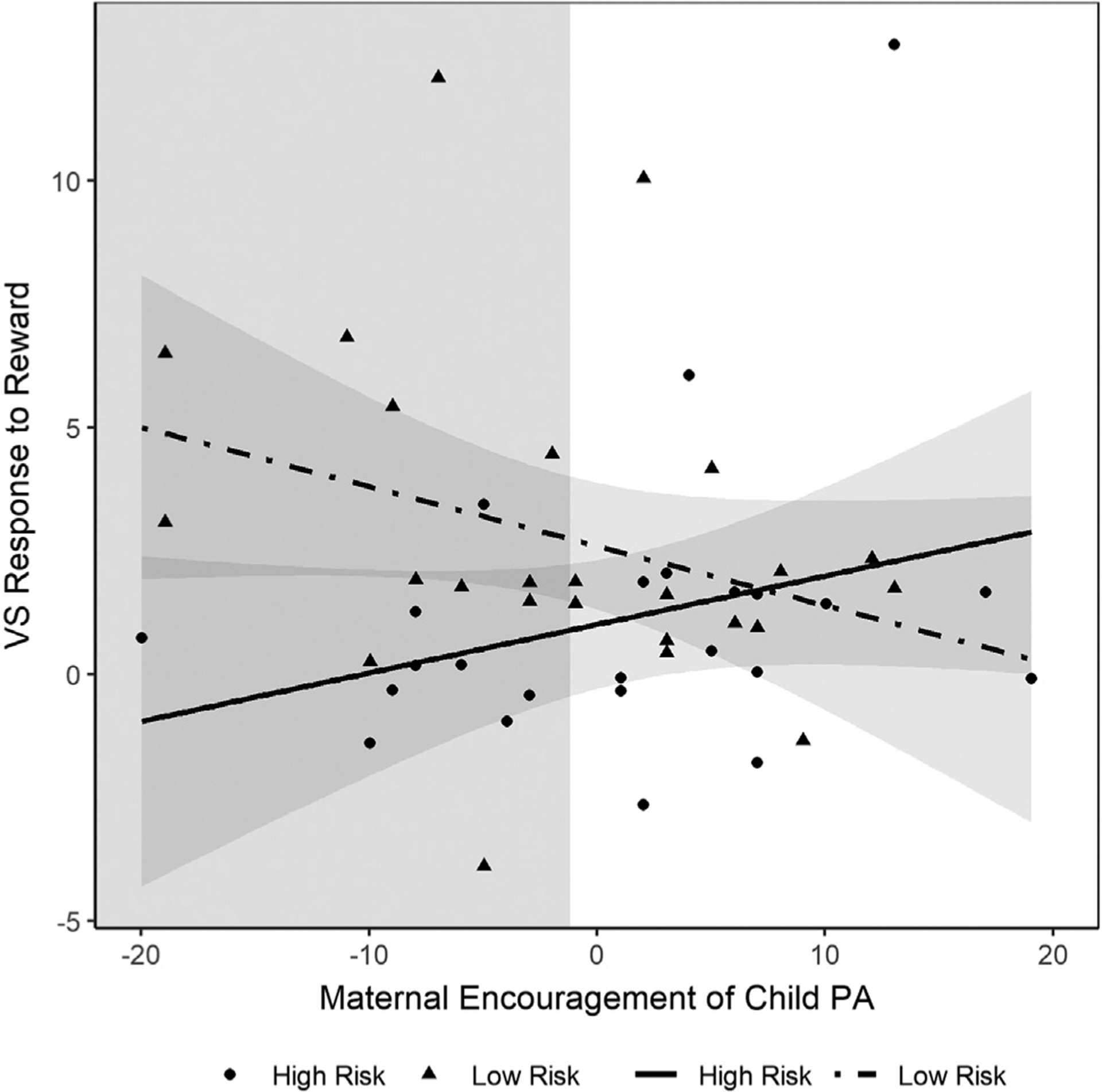
Interactive effect of maternal encouragement of child positive affect (PA) and familial risk for depression on ventral striatal (VS) activity in 6- to 8-year-old children.
Maternal Dampening of Child PA and Child VS Response.
Our second regression model revealed that risk status and maternal dampening of child PA interacted to predict VS activity (β = −0.46, t = −2.20, p = .033) (Table 5). Specifically, analysis of simple slopes revealed that high-risk children had lower VS response to reward compared with low-risk children only when maternal dampening of child PA was high (β = −0.57, t = −2.90, p = .006). When maternal dampening of child PA was low, there were no significant differences between risk groups on VS activity (β = 0.07, t = 0.34, p = .74) (Figure 3).2
Table 5.
Role of Risk Status, Maternal Dampening of Child PA, and Their Interactive Effect on Child Ventral Striatal Response to Winning Reward
| Variable | Standardized β | t 48 | p Value |
|---|---|---|---|
| Risk | −0.57 | −2.90 | .006 |
| Maternal Dampening of Child PA | 0.33 | 1.88 | .087 |
| Risk × Maternal Dampening of Child PA | −0.46 | −2.20 | .033 |
PA, positive affect.
Figure 3.
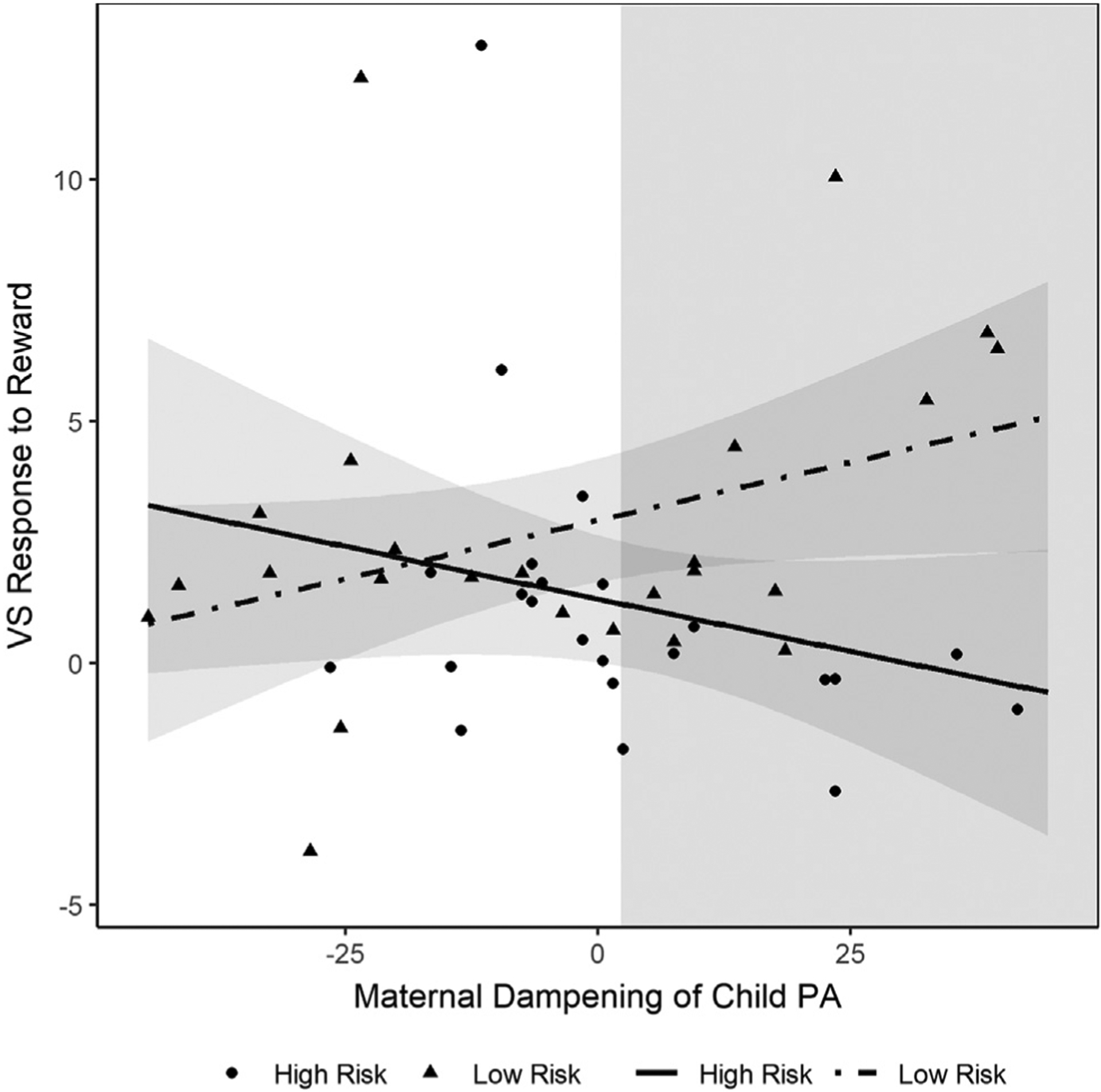
Interactive effect of maternal dampening of child positive affect (PA) and familial risk for depression on ventral striatal (VS) activity in 6- to 8-year-old children.
DISCUSSION
Our study provided evidence that children as young as 6 to 8 years at high familial risk for depression show diminished VS activity to winning reward relative to low-risk children but only in the context of either low maternal encouragement or high maternal dampening of child positive emotion expression. Our findings suggest that familial behavior patterns seem to be related to diminished reward-related neural responding even early in life, prior to the onset of puberty, when many known developmental changes in VS activity occur (21). Importantly, our findings indicate that familial risk-based alterations in VS activity appear to be present only in the context of parental emotional socialization behaviors that serve to discourage or dampen positive emotions. In this regard, associations between maternal depression and child disrupted VS response to reward were constrained to families in which mothers were less enthusiastic and responsive, and/or more dismissive or punitive during child positive emotional exchanges.
Maternal encouragement of child positive emotions, which may take the form of acknowledging, imitating, and elaborating on child positive emotion expressions (29), may reinforce activity in primary reward regions that are integral to emotional learning and play during middle childhood (23). On the other hand, maternal dampening responses, which are characterized as dismissive, invalidating, or harsh responses to child positive emotions, may, over time, limit children’s positive emotion expressions, thereby diminishing the opportunity for these reward regions to be reinforced via repeated use. Positive emotions have multiple physical and mental health–related properties, including broadening cognition, fostering exploration of new settings, and promoting social approach (4). In this regard, our findings fall in line with a large body of research demonstrating the importance of maternal positivity in child socioemotional and brain development (43). These findings also suggest that preventive interventions should be tailored to focus on maternal socialization of emotion to support healthy reward-related development in youths, especially those with a family history of depression.
Traditional behavioral parent training paradigms (e.g., Helping the Noncompliant Child) (44) often include modules in which parents are trained on how to accept, acknowledge, and coach their children through difficult emotions as well as how to provide increased attention toward their child’s positive behaviors. In addition, a new adaptation to Parent-Child Interactive Therapy that teaches parents how to serve as emotion coaches for their young children’s emotional expressions (Parent-Child Interactive Therapy–Emotional Development) includes a module in which parents are trained on how to support children’s expression of happiness/joy (45). However, many of these paradigms and interventions (including Helping the Noncompliant Child and Parent-Child Interactive Therapy–Emotional Development) often target children with existing psychopathology and focus primarily on parental socialization of child negative emotion. Our findings suggest that preventive interventions that are generalizable to children who are not yet displaying clinical disorders, but who may be at risk for developing psychopathology, and that provide a larger component focusing on socialization of positive emotion are needed.
Unexpectedly, we did not find that maternal depression was significantly associated with maternal socialization of emotion. It is important to note that although depression is associated with blunted positive emotion on average (46), there is considerable heterogeneity in positive emotion expression in individuals with depression (47). Accordingly, some mothers with depression may be more naturally encouraging of their child’s positive emotions, possibly because they have less alteration in their own reward circuitry or greater temperamental PA themselves. Additionally, there is some evidence to suggest that maternal responsivity to child positive emotions may improve with remission of maternal depression, which may have influenced the heterogeneity of maternal socialization of positive emotion in our sample. Specifically, our prior work has shown that currently depressed mothers, but not nondepressed mothers with a prior history of depression, were less likely to imitate their preschool age children’s positive emotion expressions (29).
The VS is a primary reward region that has been implicated in healthy positive emotion expression (5) and is critical to recovery from stress and psychiatric illness (4,48), which will be particularly important during the vulnerable period of adolescence. Relatedly, blunted responding in the VS in adolescents has been linked to increases in depressive symptoms across adolescence (12). Thus, VS activity appears to have important clinical and developmental functions. Understanding what factors, such as maternal emotion socialization behaviors, support healthy VS activity is important for prevention and intervention purposes.
Extensive work has already established that reward-related neural disruptions are present in adolescent offspring of depressed parents (8–11). Our findings add to a growing body of literature that these neural alterations may emerge even earlier than previously suspected (17,18,49). Because children in our study were required to be free of psychiatric illness, our findings also suggest that these neural alterations in response to winning reward appear to precede the onset of psychiatric illness and are not explained by preexisting subthreshold affective symptoms.
Future longitudinal work should evaluate how early maternal behavior and child VS response to reward predicts the onset of depression during the vulnerable period of adolescence, particularly for youths at high familial risk. Of note, we found that greater maternal dampening of child positive emotions was associated with greater child anxiety symptoms. This finding falls in line with prior work demonstrating that low levels of positive emotions are predictive of anxiety disorders (50,51). However, our design limits our ability to pinpoint directionality in this association as well as rule out the role of shared method variance (i.e., mothers reported on both dampening and child anxiety).
Our study has several strengths, including its 1) evaluation of neural response to winning reward in a very young sample of 6- to 8-year-old children, 2) examination of the association between maternal behavior and child VS activity, and 3) use of a well-characterized sample with a maternal history of recurrent or chronic depression. Its primary limitations were use of an fMRI task that contained reward but not loss trials, its focus on maternal depression versus other parental psychopathology, and its inclusion of children who were psychiatrically healthy. Future studies with larger sample sizes and that use alternative methodology to measure reward (e.g., event-related potentials) will also be needed to replicate these findings.
In summary, our findings provide evidence that children as young as 6 to 8 years who are at risk for depression may show diminished response in the VS when winning reward but only in the context of maternal discouragement of child positive emotion expression. In other words, in the context of high levels of maternal encouragement or low levels of maternal dampening of child PA expression, there were no significant differences between children at high and low familial risk for depression. This finding provides hopeful news, as this suggests that mothers’ own history of depression is insufficient to influence children’s reward circuitry, while parent behavior, which is amenable to change, appears to play an important modulatory role. Interventions geared at coaching parents to encourage positive emotions in their young children, via acknowledgement, imitation, and elaboration, may have a positive impact on child reward-related brain development, which in turn may be beneficial during the vulnerable period of adolescence.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by National Institute of Mental Health Grant No. K01MH099220 (to JKM, principal investigator).
We thank the families and staff of the CHEER (Child Happy Emotion Expression and Regulation) study at the University of Pittsburgh for their time and commitment to this research.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2021.12.014.
When we limited our within-sample t test to the 37 participants with <2-mm movement, our VS cluster remained statistically significant (159 voxels, [2, 22, −4], t = 6.01, pFWE = .028). Subsequently, regression models in which we estimated the interactive effect of risk and maternal behavior on child reward response using only the 37 participants with <2-mm movement produced substantively similar results for the interactive effects (although findings were no longer statistically significant; ps = .07–.10).
Models in which we included the SCARED and MFQ as covariates yielded substantively similar results.
REFERENCES
- 1.Gotlib IH, Goodman SH, Humphreys KL (2020): Studying the intergenerational transmission of risk for depression: Current status and future directions. Curr Dir Psychol Sci 29:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammen C, Brennan PA (2003): Severity, chronicity, and timing of maternal depression and risk for adolescent offspring diagnoses in a community sample. Arch Gen Psychiatry 60:253–258. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach RP, Admon R, Pizzagalli DA (2014): Adolescent depression: Stress and reward dysfunction. Harv Rev Psychiatry 22:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredrickson B (1998): What good are positive emotions? Rev Gen Psychol 2:300–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes EE, Dahl RE (2012): Research review: Altered reward function in adolescent depression: What, when, and how? J Child Psychol Psychiatry 53:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. (2009): Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J (2013): The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 151:531–539. [DOI] [PubMed] [Google Scholar]
- 8.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J (2010): Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry 67:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp C, Kim S, Herman L, Pane H, Reuter T, Strathearn L (2014): Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. J Abnorm Psychol 123:298–309. [DOI] [PubMed] [Google Scholar]
- 10.Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, et al. (2014): Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Dev Cogn Neurosci 8:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, et al. (2008): Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 165:90–98. [DOI] [PubMed] [Google Scholar]
- 12.Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE (2013): Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis 52:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belden AC, Irvin K, Hacjak G, Kappenman ES, Kelly D, Karlow S, et al. (2016): Neural correlates of reward processing in depressed and healthy preschool-age children. J Am Acad Child Adolesc Psychol 55:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bress JN, Smith E, Foti D, Klein DN, Hajcak G (2012): Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol Psychol 89:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kujawa A, Hajcak G, Klein DN (2019): Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: Evidence across levels of analysis. J Child Psychol Psychiatry 60:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kujawa A, Proudfit GH, Klein DN (2014): Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J Abnorm Psychol 123:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan JK, Silk JS, Woods BK, Forbes EE (2019): Differential neural responding to affective stimuli in 6-to 8-year-old children at high familial risk for depression: Associations with behavioral reward seeking. J Affect Disord 257:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiggins JL, Schwartz KT, Kryza-Lacombe M, Spechler PA, Blankenship SL, Dougherty LR (2017): Neural reactivity to reward in school-age offspring of depressed mothers. J Affect Disord 214:81–88. [DOI] [PubMed] [Google Scholar]
- 19.Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC (2005): Temperamental emotionality in preschoolers and parental mood disorders. J Abnorm Psychol 114:28–37. [DOI] [PubMed] [Google Scholar]
- 20.Olino TM, Lopez-Duran NL, Kovacs M, George CJ, Gentzler AL, Shaw DS (2011): Developmental trajectories of positive and negative affect in children at high and low familial risk for depressive disorder. J Child Psychol Psychiatry 52:792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galván A (2013): The teenage brain: Sensitivity to rewards. Curr Dir Psychol Sci 22:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thapar A, Collishaw S, Pine DS, Thapar AK (2012): Depression in adolescence. Lancet 379:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson EE, Jarcho JM, Guyer AE (2016): Social re-orientation and brain development: An expanded and updated view. Dev Cogn Neurosci 17:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan PZ, Oppenheimer CW, Ladouceur CD, Butterfield RD, Silk JS (2020): A review of associations between parental emotion socialization behaviors and the neural substrates of emotional reactivity and regulation in youth. Dev Psychol 56:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR (2007): The role of the family context in the development of emotion regulation. Soc Dev 16:361–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow KE, Gentzler AL, Wilson TK, Room KF, Root AE (2021): Maternal depression and socialization of children’s positive affect regulation. J Child Fam Stud 187:106–113. [Google Scholar]
- 27.Feldman R (2015): The adaptive human parental brain: Implications for children’s social development. Trends Neurosci 38:387–399. [DOI] [PubMed] [Google Scholar]
- 28.Harrist AW, Waugh RM (2002): Dyadic synchrony: Its structure and function in children’s development. Dev Rev 22:555–592. [Google Scholar]
- 29.Morgan JK, Silk JS, Olino TM, Forbes EE (2020): Depression moderates maternal response to preschoolers’ positive affect. Infant Child Dev 29:e2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yap MB, Allen NB, Ladouceur CD (2008): Maternal socialization of positive affect: The impact of invalidation on adolescent emotion regulation and depressive symptomatology. Child Dev 79:1415–1431. [DOI] [PubMed] [Google Scholar]
- 31.Morgan JK, Shaw DS, Forbes EE (2014): Maternal depression and warmth during childhood predict age 20 neural response to reward. J Am Acad Child Adolesc Psychol 53:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kujawa A, Proudfit GH, Laptook R, Klein DN (2015): Early parenting moderates the association between parental depression and neural reactivity to rewards and losses in offspring. Clin Psychol Sci 3:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladouceur C, Reid L, Jacques A (2002): Construction et validation du Questionnaire sur les reactions parentales aux emotions positives exprimees par l’enfant. Can J Behav Sci 34:8–18. [Google Scholar]
- 34.Gentzler AL, Ramsey MA, Black KR (2015): Mothers’ attachment styles and their children’s self-reported security, as related to maternal socialization of children’s positive affect regulation. Attach Hum Dev 17:376–398. [DOI] [PubMed] [Google Scholar]
- 35.Moran KM, Root AE, Vizy BK, Wilson TK, Gentzler AL (2019): Maternal socialization of children’s positive affect regulation: Associations with children’s savoring, dampening, and depressive symptoms. Soc Dev 28:306–322. [Google Scholar]
- 36.Yi CY, Gentzler AL, Ramsey MA, Root AE (2016): Linking maternal socialization of positive emotions to children’s behavioral problems: The moderating role of self-control. J Child Fam Stud 25:1550–1558. [Google Scholar]
- 37.Angold A, Costello EJ, Messer SC, Pickles A (1995): Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res 5:237–249. [Google Scholar]
- 38.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M (1999): Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. J Am Acad Child Adolesc Psychiatry 38:1230–1236. [DOI] [PubMed] [Google Scholar]
- 39.Foti D, Carlson JM, Sauder CL, Proudfit GH (2014): Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage 101:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luking KR, Nelson BD, Infantolino ZP, Sauder CL, Hajcak G (2017): Internal consistency of functional magnetic resonance imaging and electroencephalography measures of reward in late childhood and early adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging 2:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsypes A, Gibb BE (2020): Time of day differences in neural reward responsiveness in children. Psychophysiology 57:e13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter CS, Lesh TA, Barch DM (2016): Thresholds, power, and sample sizes in clinical neuroimaging. Biol Psychiatry Cogn Neurosci Neuroimaging 1:99–100. [DOI] [PubMed] [Google Scholar]
- 43.Teti DM, Cole PM, Cabrera N, Goodman SH, McLoyd VC (2017): Supporting parents: How six decades of parenting research can inform policy and best practice. Soc Policy Rep 30:1–34. [Google Scholar]
- 44.McMahon RJ, Forehand RL (2005): Helping the Noncompliant Child: Family-Based Treatment for Oppositional Behavior. New York: Guilford Press. [Google Scholar]
- 45.Luby J, Lenze S, Tillman R (2021): A novel early intervention for preschool depression: Findings from a pilot randomized controlled trial. J Child Psychol Psychiatry 53:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderlind WM, Millgram Y, Baskin-Sommers AR, Clark MS, Joormann J (2020): Understanding positive emotion deficits in depression: From emotion preferences to emotion regulation. Clin Psychol Rev 76:101826. [DOI] [PubMed] [Google Scholar]
- 47.Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, et al. (2018): Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J Affect Disord 231:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sequeira SL, Silk JS, Ladouceur CD, Hanson JL, Ryan ND, Morgan JK, et al. (2021): Association of neural reward circuitry function with response to psychotherapy in youths with anxiety disorders. Am J Psychiatry 178:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luking KR, Pagliaccio D, Luby JL, Barch DM (2016): Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. J Am Acad Child Adolesc Psychiatry 55:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan JK, Lee GE, Wright AGC, Gilchrist DE, Forbes EE, McMakin DL, et al. (2017): Altered positive affect in clinically anxious youth: The role of social context and anxiety subtype. J Abnorm Child Psychol 45:1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rackoff GN, Newman MG (2020): Reduced positive affect on days with stress exposure predicts depression, anxiety disorders, and low trait positive affect 7 years later. J Abnorm Psychol 129:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


