Abstract
Background: Allogeneic stem cell transplantation (allo-SCT) is the highest potential treatment for long-term survival as post-remission therapy for acute myeloid leukemia (AML). The aim of this study was to estimate the overall survival (OS) of patients with AML after allo-SCT and to identify the factors affecting them as a prognostic factor for the survival of patients.
Material and Methods: In this retrospective cohort study, data of patients with AML who underwent allo-SCT at Taleghani bone marrow transplantation and cell therapy center in Tehran, Iran, from May 2009 to September 2016 were used. A total of 101 patients were enrolled and death time was considered as a failure event for them. Kaplan-Meier method, log-Rank tests, and Cox proportional hazard model were used to evaluate OS and to identify the risk factors of patient’s survival. The SPSS software version 21 was used for the analysis of data and P<0.05 was considered as a significant level.
Results: Of 101 patients with AML, 49 (48.5%) were males. The median age at allo-SCT was 32.76 years and 42 patients (41.6%) died. The 5-year OS and disease-free survival (DFS) was 56% (95%CI: 51-61%) and 52% (95%CI: 57-47%), respectively. Multivariate analysis by Cox regression indicated that OS has a significant relationship with primary WBC count and relapse (P=0.001).
Conclusion: Our results showed that allo–SCT has nearly the same outcome in developing countries and the WBC count and relapse are effective factors on the chance of survival in AML patients after allo-SCT.
Key Words: Acute myeloid leukemia, Allogeneic stem cell transplantation, Survival analysis, Prognostic factors
Introduction
Acute myeloid leukemia (AML) a cancer of myeloid cells in the bone marrow is the most common acute leukemia in the adult population1,2. Without treatment, AML is typically fatal within weeks to months 3. The burden of AML became heavier during the past 28 years, which might need more health resource especially in developing countries who had the most AML incidences and deaths4. The goal of AML therapy is to achieve and sustain remission, and treatment conventionally consists of two phases. In the first phase of treatment, the goal is to induce remission with conventional-dose of chemotherapy5. After achieving a first complete remission (CR1), second phase of therapy is mandatory to prevent relapse 6,7. Post-remission therapy in patients with AML may consist of continuing chemotherapy or transplantation using either autologous or allogeneic stem cells8. Data on cytogenetic abnormalities and somatic mutations has provided important information that is essential for therapeutic decision making9 . Patients with AML can be classified into three prognostic groups based on cytogenetic; good, intermediate, and high-risk [10,11]. Therefore, the intensity and type of post-remission therapy is usually determined by cytogenetic and molecular factors 6,12. Chemotherapeutic consolidation is usually recommended for good-risk genetic subtypes of AML and allogeneic hematopoietic stem cell transplantation (allo-SCT) for intermediate and high-risk genetic subtypes of AML 13.
Allo-SCT is the highest potential treatment for long-term survival as post-remission therapy in those with an intermediate or high-risk subtype and as salvage therapy in those with relapsed or resistant disease14,15. The Center of International Blood and Marrow Transplant Research (CIBMTR) and the National Marrow Donor Program (NMPD) have been reported 65% survival rates in AML patients after stem cell transplantation, while the 5-year survival rate of adult subjects with AML without allo-SCT is approximately 24% 16-18. However, the benefit of allo-SCT in overall survival may be compromised by relapse and treatment-related mortality (TRM) 19-21. Relapse is the major cause of treatment failure and up 50% of AML patients finally relapse after allo-SCT depending on disease status and characteristics [22]. According to the CIBMTR, relapse rates following HLA-matched transplantation range was reported from approximately 25% and 60% for AML patients in CR1 and high-risk subtype patients, respectively23. A graft-versus-leukemia effect gives allo-SCT superior anti-leukemic activity, with a greater chance of maintaining remission than is achieved with consolidation chemotherapy 24,25. However, its benefit is limited by greater TRM, which can be as high as 20%–30%, and the morbidity and mortality associated with graft-versus-host disease (GVHD)26,27.
Estimate the survival rate is very useful in monitoring and improving the quality of life of patients with AML, which can lead to conducting better screening programs and discovering new treatments. We conducted this study to evaluate the 5-year overall survival (OS) and disease-free survival (DFS) rates of patients with AML after allo-SCT and to identify the factors affecting them as a prognostic factor for the survival of AML patients, who referred to bone marrow transplantation and cell therapy center at Taleghani Hospital, Tehran, Iran.
MATERIALS AND METHODS
Study design and participants
All patients with AML who underwent a first allo-SCT from May 2009 to September 2016 at bone marrow transplantation and cell therapy center at Taleghani Hospital, Tehran, Iran were encludeded in the retrospective cohort study. AML patients who underwent autologous bone marrow transplantation were excluded from this study. All demographic characteristics and clinical data of patients and donor of stem cells were obtained. Age at transplantation, sex, date of initial AML diagnosis, AML subtype ,disease status at transplantation time(CR1 or CR2), date of allo-SCT, sibling or alternative related donor, age of donor, blood type of patient and white blood cell (WBC) counts of patients at diagnosis were obtained. Furthermore, the post-transplant variables and outcomes such as; relapse status, date of last follow-up or death, and the cause of death were recorded. Approval for this study was conducted by the Ethical Committee of Taleghani bone marrow transplantation and cell therapy center of Shahid Beheshti University of Medical Science and the study registered code in Shahid Beheshti School of Medicine is 139514. Written informed consent was obtained from all patients.
Preparative regimen
All patients receive a Bu/Cy regimen (Busulfan 4 mg/kg/day orally or 3.2 mg/kg/day on days -6 to -3 and Cyclophosphamide 60 mg/kg/day by intravenous infusion on days -2 to -1) or BU/Fu (Busulfan 3.2 mg/kg/day intravenously ,on days -7 to -4 and Fludarabine 50mg/m2/day by intravenous infusion on days -5 to -1) for conditioning therapy with subsequent infusion of donor marrow cells on day 0.
Definition
Remission status post-chemotherapy was documented on the basis of criteria laid down by Cheson et al. [28] and European Leukemia Net. [29] Primary induction failure was defined as patients who experienced a failure to achieve remission after two induction chemotherapies. CR1 was defined as remission achieved within one or two consecutive induction chemotherapy regimens. CR2 was defined as remission after receiving salvage chemotherapy for first relapse.
Relapse: Relapse was defined as a recurrence of leukemia confirmed by cytology. Overall Survival (OS) was defined as the time interval between allo-SCT and death of any cause or censoring. DFS was defined as the time from allo-SCT to death without any relapse. Censoring was defined as being alive at the last follow-up.
Statistical analysis
Frequency tables including number and percent were prepared for qualitative variables also mean and standard deviation (Mean ± SD) were presented for continuous variables. OS and DFS of patients were the end points of study. OS was defined as the time interval between allo-SCT and death from any cause related to AML. DFS was defined as the time from allo-SCT to death without any relapse. Censoring was defined as being alive at the last follow-up. Survival after allo-SCT was calculated using the Kaplan–Meier (KM) curve, and the log-rank test was used to compare survival probability between groups. Univariate and multivariate Cox proportional hazard regression was used to determine the prognostic factors of OS and DFS. The Cox proportional assumptions for hazard were not met in defining the relationship between different types of AML based on FAB categories and survival, we only compare three types of AML with high frequency in this study M4, M5 and M2. Hazard ratio (HR) and 95% confidence interval (CI) were reported to compare hazards between patients groups. For all tests, P≤0.05 was considered significant. Statistical analysis was performed using SPSS for windows version 21 (SPSS, Chicago, IL).
Results
Patients characteristic
Between May 2009 and September 2016, 101 consecutive patients with AML in first or second complete remission (CR1/CR2) received allo-SCT from human leukocyte antigen (HLA)-identical matched sibling or non-sibling. The mean age of patients at transplant time was 32.76 ± 9.23 years (range: 3–53 years), and 49 of them (48.5%) were men. Among of our cases with AML, the subgroups frequency of M0, M1, M2, M3,M4, M5 and M6 were 1%, 7.9%, 18.8%, 4%, 36.6%, 29.7%, and 2%, respectively. Most of the patients (97%) underwent transplanted in CR1 and only (3%) of them were transplanted in CR2. Cause of death (n=42) included infection (n=20, 47.6%), GVHD (n=12, 28.6%) and leukemia relapse (n=10. 23.8%). Patients and transplant characteristics are presented in Table 1.
Table 1.
Patients and transplant characteristics
| Characteristics | Frequency (%) | |
|---|---|---|
| Sex | Male | 49 (48.5) |
| Female | 52 (51.5) | |
| Age at transplant | (Mean ± SD) | 32.76 ± 9.23 |
| (Range) | 5-53 | |
| Age group | ≤ 30 | 44 (43.6) |
| >30 | 57 (56.4) | |
| Diseases status at transplant |
CR1∞ | 98(97.0) |
| CR2 | 3(3) | |
| Blood group | A+ | 29(28.7) |
| B+ | 15(14.9) | |
| O+ | 37(36.6) | |
| O- | 7(6.9) | |
| AB+ | 13(12.9) | |
| WBC (×108 /ml) | <5 | 17(16.8) |
| 5-10 | 15(14.9) | |
| >10 | 52(51.5) | |
| unknown | 17(16.8) | |
| M3 AML | M3 | 4(4.0) |
| Non-M3 | 97(96.0) | |
| All types of ALM | M0 | 1(1.0) |
| M1 | 8(7.9) | |
| M2 | 19(18.8) | |
| M3 | 4(4.0) | |
| M4 | 37(36.6) | |
| M5 | 30(29.7) | |
| M6 | 2(2.0) | |
| Relapse | Yes | 13(12.9) |
| No | 88(87.1) | |
| Cause of death | Infection | 20 (47.6) |
| GVHD | 12 (28.6) | |
| Relapse | 10 (23.8) | |
Donors characteristic
The mean age of donors at transplant time was 33.39 ± 10.71 years (range: 5–56 years) and 54 of them (53.5%) were male. In 92 patients (91.1%), the allo-SCT donation came from a sibling and in 9 patients (8.9%) it came from a related alternative donor (a double cousin). For 66 of the recipient–donor pairs (65.3%), an ABO blood group match was attained, 21 pairs (20.8%) had a minor ABO mismatch, and 14 pairs (13.9%) had a major ABO mismatch. In 50 patients (49.5%), the recipient–donor pairs were sexually matched while in others 51 (50.5%) was not. Of the 59 male recipients, 28 (47.4%) had a female donor, and of the 52 female recipients, 23 (44.2%) had a male donor. The main characteristics of donors are presented in Table 2.
Table 2.
Characteristics of donors
| Characteristics | Frequency (%) | |
|---|---|---|
| Sex | Male | 54(53.5) |
| Female | 47(46.5) | |
| Age | Mean ± SD | 33.39 ±10.71 |
| Range | 5-56 | |
| Age group | ≤ 30 | 40 (39.6) |
| > 30 | 61 (60.4) | |
| sex of donor / recipient | Male/Female | 28(27.7) |
| Male/Male | 26(25.7) | |
| Female/Female | 24(23.8) | |
| Female/Male | 23(22.8) | |
| Blood group | A+ | 25(24.8) |
| A- | 1(1.0) | |
| B+ | 27(26.7) | |
| O+ | 36(35.6) | |
| O- | 4(4.0) | |
| AB+ | 8(7.9) | |
| Donor relatives | Sibling | 92(91.1) |
| Related alternative | 9(8.9) | |
Survival analysis
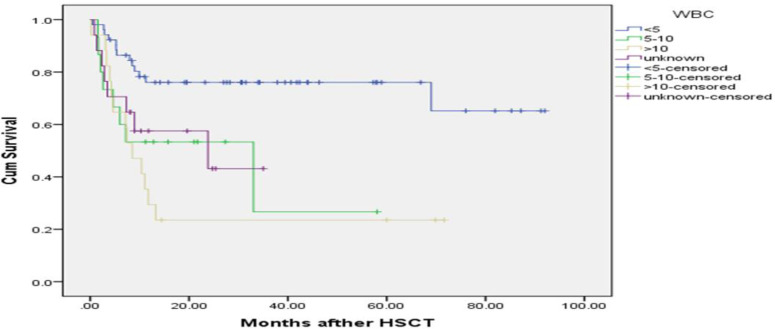
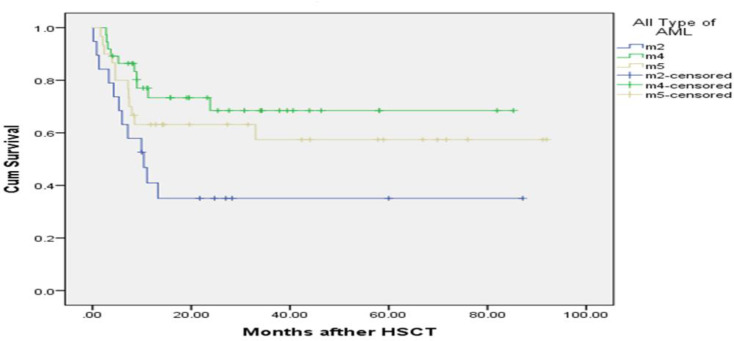
The median follow up time was 13.16 months (mean: 24.09±2.41 months). Up to the end of the study, 42 (41.6%) patients died and 59 patients (58.4%) were censored. The mean survival time in patients was 53.70 months (95% CI: 44.89-62.51) and for DFS was 50.80 (95% CI: 41.87-59.73). The 1, 3 and 5-year OS was 60% (95% CI: 51-69%), 58% (95% CI: 51-64%) and 56% (95% CI: 51-61%), respectively. The 1, 3 and 5-year DFS was 58% (95% CI: 53-63%), 54% (95% CI: 49-60%) and 52% (95% CI: 47-57%), respectively. The KM curves of OS for comparing sex, age, relapse, WBC counts and different types of AML (M2, M4 and M5) are shown in Figures 1 to 3. The survival time was compared among groups according to the Log-Rank test. There is a statistical difference between categories of WBC counts (P=0.001) and relapse of leukemia (P=0.006) and the different types of AML (M4, M5 and M2) (P=0.03) in OS. The number of patients with post-transplant relapse of leukemia was 13 (12.9%) and the mean time from allo-SCT to relapse for them was 4.87±3.9 months (range: 0.9-14.73). Complete remission after allo-SCT was achieved in 56 (55.4%) patients.
Figure 1.
Kaplan-Meier estimated overall survival of AML patients after allo-SCT according to WBC counts, there was a strong correlation between OS and WBC counts of patients after transplantation (P=0.001), those patients with WBC more than (10×108/ml), their OS was about 4.06 times shorter than the others.
Univariate analysis for prognostic factors
Univariate analysis showed a significant association between OS with WBC counts (P=0.001), relapse (P=0.008) and AML-M2 (P=0.004). There was no any significant association between other variables with OS. The hazard ratio (HR) and corresponding CIs for OS are presented in Table 3. The estimated hazard of death was 4.06 (95% CI, 1.88-8.80) for WBC more than (10×108/ml) and 2.97 (95% CI, 1.23-7.19) for WBC (5-10×108/ml), indicating the adverse effect of this variable on survival time. Hazard of death for relapse was estimated to be 2.61 (95% CI, 1.28-5.34). This rate indicates the power of relapse in decreasing
Table 3.
Prognostic Factors of overall survival (OS) in patients with AML after
|
Variable
|
Survival time
|
Univariate
|
Multivariate
|
|||
|---|---|---|---|---|---|---|
| Mean ± SD (Months) |
HR (95% CI) |
P-value | HR (95% CI) |
P-value | ||
| Sex | Male | 58.06 ± 6.38 | 0.76 (0.41-1.40) | 0.38 | ||
| Female | 42.97 ± 4.91 | 1 | ||||
| Age group of patients | ≤ 30 | 52.69 ± 6.76 | 1.11 (0.61-2.04) | 0.73 | ||
| > 30 | 53.98 ± 5.94 | 1 | ||||
| Diseases status at transplant |
CR1 | 54.34 ± 4.56 | 0.69 (0.17-2.87) | 0.61 | ||
| CR2 | 20.52 ± 8.71 | 1 | ||||
| WBC (×108 /ml) | <5 | 68.94 ± 5.82 | 1 | 1 | ||
| 5-10 | 26.00 ± 6.8 | 2.97 (1.23-7.19) | 0.02* | 2.76 (1.13-6.72) | 0.03* | |
| >10 | 22.09 ± 6.77 | 4.06 (1.88-8.80) | <0.001* | 3.98 (1.84-8.63) | <0.001* | |
| Donor relative | Sibling | 55.91 ± 4.67 | 0.48(0.20-1.14) | 0.09 | ||
| Related alternative | 14.63 ± 4.61 | 1 | ||||
| Relapse | Yes | 25.11 ± 9.45 | 2.61 (1.28-5.34) | 0.008* | 5.66 (2.6-12.34) | <0.001* |
| No | 57.94 ± 4.79 | 1 | 1 | |||
| Age group of donors | ≤ 30 | 58.13 ± 6.86 | 0.82 (0.43-1.54) | 0.53 | ||
| > 30 | 48.15 ± 5.51 | 1 | ||||
| Sex of donor - recipient | F-F | 47.70 ± 7.11 | 1.35 (0.58-3.14) | 0.48 | ||
| F-M | 59.45 ± 9.21 | 0.91 (0.36-2.32) | 0.85 | |||
| M-F | 42.27 ± 6.37 | 1.18 (0.51-2.73) | 0.70 | |||
| M-M | 57.3 ± 8.28 | 1 | ||||
| AML | M3 | 20.55 ± 7.49 | 1.15(0.28-4.76) | 0.84 | ||
| Non-M3 | 54.00 ± 4.58 | 1 | ||||
| Different types of ALM | M2 | 34.62 ± 9.11 | 10.3 (0.13-0.68) | 0.04* | 0.56 (0.21-1.49) | 0.25 |
| M4 | 61.24 ± 6.43 | 1 | 1 | |||
| M5 | 56.66 ± 7.89 | 0.51 (0.23-1.10) | 0.09 | 0.72 (0.32-1.61) | 0.42 | |
Acute graft-versus-host diseases (aGVHD), chronic graft-versus-host diseases (cGVHD), first and second complete remission (CR1, CR2)
the survival time. Hazard of death for AML-M2 was estimated to be 10.3 (95% CI, 0.13-0.68). This rate indicates that the patients with AML-M2 had a shorter overall survival time. In addition, univariate analysis showed a significant association between DFS with WBC counts (P=0.001) and AML-M2 (P=0.004). The estimated hazard of death was 3.57 (95% CI, 1.69-7.56) for WBC more than (10×108/ml) and 2.9 (95% CI, 1.26-6.67) for WBC (5-10×108/ml), indicating the adverse effect of this variable on disease-free survival time. Hazard of death for AML-M2 was estimated to be 0.30 (95% CI, 0.13-0.68). This rate indicates the power of the AML-M2 variable in decreasing disease-free survival time. The HR and corresponding CIs for DFS are presented in Table 4.
Table 4.
Prognostic Factors of disease-free survival (DFS) in patients with AML after allo-SCT
|
Variable
|
Survival time
|
Univariate
|
Multivariate
|
|||
|---|---|---|---|---|---|---|
| Mean ± SD (Months) |
HR (95% CI) |
P-value | HR (95% CI) |
P-value | ||
| Sex | Male | 51.95 ± 6.62 | 0.91 (0.51-1.64) | 0.76 | ||
| Female | 42.87 ± 4.94 | 1 | ||||
| Age group of patients | ≤ 30 | 50.60 ± 6.79 | 1.06 (0.59-1.91) | 0.84 | ||
| > 30 | 50.13 ± 6.11 | 1 | ||||
|
Diseases status at
transplant |
CR1 | 51.33 ± 4.63 | 0.64 (0.15-2.65) | 0.54 | ||
| CR2 | 18.36 ± 9.64 | 1 | ||||
| WBC (×10 8 /ml) | <5 | 65.15 ± 5.82 | 1 | 1 | ||
| 5-10 | 23.21 ± 6.80 | 2.9 (1.26-6.67) | 0.02* | 0.39(0.15-1.00) | 0.07 | |
| >10 | 21.50 ± 6.77 | 3.57 (1.69-7.56) | 0.001* | 0.86 (0.28-2.62) | 0.05 | |
| Donor relative | Sibling | 52.73 ± 4.75 | 0.56 (0.23-1.32) | 0.18 | ||
| Non- sibling | 14.63 ±4.61 | 1 | ||||
| Age group of donors | ≤ 30 | 55.82 ± 6.97 | 0.82 (0.45-1.52) | 0.53 | ||
| > 30 | 43.88 ± 5.43 | 1 | ||||
|
Sex of donor –
recipient |
F-F | 41.56 ± 7.17 | 1.35 (0.58-3.13) | 0.48 | ||
| F-M | 46.82 ± 9.59 | 1.28 (0.54-3.0) | 0.58 | |||
| M-F | 42.36 ± 6.37 | 1.14 (0.49-2.64) | 0.76 | |||
| M-M | 57.35 ± 8.39 | 1 | ||||
| AML | M3 | 20.55 ± 7.49 | 1.15 (0.28-4.76) | 0.84 | ||
| Non-M3 | 50.99 ± 4.46 | 1 | ||||
|
Different types of
ALM |
M2 | 22.03 ± 5.89 | 0.30 (0.13-0.68) | 0.04* | 0.43 (0.17-1.11) | 0.08 |
| M4 | 61.54 ± 6.37 | 1 | 1 | |||
| M5 | 52.84 ± 8.14 | 0.51 (0.23-1.10) | 0.08 | 0.57 (0.26-1.27) | 0.17 | |
Acute graft-versus-host diseases (aGVHD), chronic graft-versus-host diseases (cGVHD), first and second complete remission (CR1, CR2)
Multivariate analysis for prognostic factors
In a multivariate model, OS had a strong association with WBS counts (P=0.001) and relapse (P=0.001) (Table 3). The estimated hazard of death was 3.98 (95% CI, 1.84-8.68) for WBC more than (10×108/ml) and 2.76 (95% CI, 1.13-6.72) for WBC (5-10×108/ml), indicating the adverse effect of this variable on survival time. Hazard of death for relapse was estimated to be 5.66 (95% CI, 2.6-12.34). This rate indicates the power of relapse in decreasing the survival time. Moreover, multivariate cox model only revealed a significant association for WBC counts (P=0.001) with DFS (Table 4). The estimated hazard of death was 3.80 (95% CI, 1.78-8.10) for WBC more than (10×108/ml) and 2.58 (95% CI, 1.11-5.97) for WBC (5-10×108/ml), indicating the adverse effect of this variable on disease-free survival time.
Figure 2.
Kaplan-Meier estimated overall survival of AML patients after allo-SCT according to different types of AML (M2, M4 and M5), there was a correlation between OS and the type of AML of patients after transplantation (P=0.02), those patients with AML-M2, their OS was about 10.3 times shorter than the others (AML-M4 and AML-M5).
Discussion
In the present retrospective cohort study, we studied outcomes of 101 AML patients, who underwent allo-SCT (2009–2016) in CR1 or CR2 to determine the 5-year post-transplant OS, DFS and identify pre and post-transplant factors associated with survival rate. Our results indicated the 5-year post-transplant OS and DFS 56% (95% CI: 51-61%) and 52% (95% CI: 47-57%), respectively. The 5-year survival rate reported in our study is similar to previously reported by Mitus et al. [30] that reported the 5-year OS 55% and 3-year survival calculated by Baron et al. [31] was 54±1%. The results showed that OS and DFS of patients after allo-SCT were reduced with time and the results of the other studies confirm our findings. In a retrospective cohort study by Frazer et al. on 55 AML patients showed 60%, 45.5%, and 37.5% OS at 1, 3, and 5 years post-transplant, respectively [32].
In previous studies, the mean survival time in AML patients who did not have a history of transplantation were reported between 14 and 17 months 33,34.. While in our study, the mean survival time in AML patients was 53.70 months (95% CI: 44.89-62.51) and for DFS was 50.80 (95% CI: 41.87-59.73). According to the CIBMTR and the NMPD have been reported 65% survival rates in AML patients after stem cell transplantation 16. Thus, improvement in the survival of AML patients with allo-SCT seems to be similar in our center.
Various studies, including meta-analysis, suggest that allo-SCT in CR1 is the best option for consolidation in high and intermediate-risk patients with AML [21,35,36]. In addition, many studies have shown improved OS (in the range of 10% –15% at 5 years) in patients, who underwent allo-SCT in CR137,38. In a retrospective descriptive study by Ganapule et al. on 254 patients with AML who underwent allo-SCT, the 5-year OS for the CR1 and CR2 was 53.1 ± 5.2% and 48.2 ± 8.3%, respectively36. The study of Sayehmiri et al. have shown the statistically significant of 5-year survival rates in AML patients at CR1 than CR2 (53% vs. 48.2%, P=0.001) [39]. In the present study, majority of patients (97%) received allo-SCT at CR1 and the mean survival time in these patients was (54.34 ± 4.56) months and it is comparable to rates published in other studies 32,40. On the other hand, the mean survival time in patients who received allo-SCT at CR2 in our center was (20.52 ± 8.71) months. However, we observed no statistical significant differences between them (P=0.61). It could be due to very low percentage of patients who received the transplant in CR2 (3%).
In the present study multivariate analysis by Cox regression indicated that, OS has significant relationship with WBC count and relapse (P=0.001). Multiple studies have shown that the high WBC count at presentation is an unfavorable prognostic factor for treatment outcome in AML patients 41-44. According to previous studies, our analysis revealed that WBC count had a significant impact on OS and DFS (P<0.001) in AML patients. So, our results demonstrate that high WBC count is prognostic factor in patients with AML. AML patients with a WBC count less than 5×108 /ml or between 5–10×108 /ml had a relatively favorable prognosis with a mean OS of (68.94 ± 5.8) and (26.00 ± 6.8) months, respectively. In contrast, patients with a WBC greater than 10×108 /ml evidently had a poor prognosis with a mean OS of (22.09 ± 6.77) months and hazard of death was 3.98 times higher in these patients.
Age of patients at transplant time is another prognostic factor associated with survival 45,46. Altered disease biology and adverse prognostic cytogenetic are more frequently associated with increased age, and older adults are often unable to tolerate further intensive therapy, and are more likely to be managed supportively 47,48. The mean age at transplant time in our population was 32.76 ± 9.23 years (range: 3–53 years). In the current study, 97% of patients were age <40 years at the time of allo-SCT. No significant relationship between age at transplant time and survival was detected (P=0.73) in the study, It might be due to overall young age of study group which is consistent with the similar study by Shokouhi et al. on 587 AML patients with a mean age of 27.27 ± 12.45 years at transplant time49. Most studies have shown that people over the age of 60 years are less likely to survive than younger people 50,51.
AML relapse after allo-SCT predicted poor survival. It remains as a major therapeutic challenge in AML patients. Bejanyan et al. studied outcomes of 1788 AML patients relapsing after allo-SCT (1990–2010) during CR1 or CR2 to identify factors associated with longer post-relapse survival and median time of post-transplant relapse was 7 months (range: 1–177)40. A study by the European Blood and Marrow Transplantation (EBMT) group showed cumulative incidence of relapse after allo-SCT 32% ± 1% among AML patients and the longer intervals from transplant to relapse, low bone marrow tumor burden at relapse, and the absence of aGVHD were identified as prognostic factors associated with survival improvement in these patients 52. Similar to prior reports we observed poor survival following AML relapse after allo-SCT 49,53. OS had a strong relationship with leukemia recurrence (P<0.001). The results indicate that a hazard of death score in patients who had relapsed was 5.66 times worse than patients who had not relapsed.
CONCLUSION
In conclusion, we conducted this retrospective cohort study to determine the 5-year post-transplant OS, DFS and identify pre and post-transplant factors associated with survival rate. Our results indicated the 5-year post-transplant OS and DFS 56% and 52%, respectively which is a considerable outcome for our patients. Analysis by Cox regression indicated that the WBC count and relapse are effective factors on the chance of survival in AML patients after allo-SCT. Absence of the cytogenetic and molecular studies are the most important limitations of this study. Patients referred to our center from different part of the country and these tests were not available or covered financially in their governmental hospitals . Based on the importance of cytogenetic data in AML patients, we recommend to perform the tests before chemotherapy to find the best personalized treatment for each patient.
Acknowledgment
This study has been supported by Taleghani Bone Marrow Transplantation Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The authors thank all staff of Taleghani Hospital and all patients for helping and participating in the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Saultz JN, Garzon R. Acute Myeloid Leukemia: A Concise Review. J Clin Med. 2016;5(3):33. doi: 10.3390/jcm5030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28(17):2859–67. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khwaja A, Bjorkholm M, Gale RE, et al. Acute myeloid leukaemia. Nat Rev Dis Primers . 2016;2:16010. doi: 10.1038/nrdp.2016.10. [DOI] [PubMed] [Google Scholar]
- 4.Ming Yi, Anping Li, Linghui Zhou, et al. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. 2020;13(1):72. doi: 10.1186/s13045-020-00908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Kouchkovsky I, Abdul-Hay M. 'Acute myeloid leukemia: a comprehensive review and 2016 update'. Blood Cancer J. 2016;6(7):e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rautenberg C, Germing U, Haas R, et al. Relapse of Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: Prevention, Detection, and Treatment. Int J Mol Sci. 2019;20(1):228. doi: 10.3390/ijms20010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CJ, Savani BN, Mohty M, et al. Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: expert review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2019;54(4):519–530. doi: 10.1038/s41409-018-0286-2. [DOI] [PubMed] [Google Scholar]
- 8.Schlenk RF. Post-remission therapy for acute myeloid leukemia. Haematologica. 2014;99(11):1663–70. doi: 10.3324/haematol.2014.114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett AK. Optimal induction and post-remission therapy for acute myeloid leukemia. Leuk Suppl . 2012;1(Suppl 2):S14–5. doi: 10.1038/leusup.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders MA, Valk PJ. The evolving molecular genetic landscape in acute myeloid leukaemia. Curr Opin Hematol . 2013;20(2):79–85. doi: 10.1097/MOH.0b013e32835d821c. [DOI] [PubMed] [Google Scholar]
- 11.Gupta M, Mahapatra M, Saxena R. Cytogenetics' impact on the prognosis of acute myeloid leukemia. J Lab Physicians . 2019;11(2):133–137. doi: 10.4103/JLP.JLP_164_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai Y. [AML treatment strategy based on cytogenetic abnormalities and somatic mutations]. [Rinsho ketsueki] Rinsho Ketsueki. 2015;56(10):1932–41. doi: 10.11406/rinketsu.56.1932. [DOI] [PubMed] [Google Scholar]
- 13.Lagunas-Rangel FA, Chavez-Valencia V, Gomez-Guijosa MA, et al. Acute Myeloid Leukemia-Genetic Alterations and Their Clinical Prognosis. Int J Hematol Oncol Stem Cell Res . 2017;11(4):328–339. [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer . 2011;2(2):95–107. doi: 10.1177/1947601911408076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Othus M, Appelbaum FR, Petersdorf SH, et al. Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol Blood Marrow Transplant. 2015;21(3):559–64. doi: 10.1016/j.bbmt.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolb E Anders, Pooja Gidwani, Stephan A Grupp. Hematopoietic Stem Cell Transplantation. eMedicine Specialties 2006. Pediatrics: General Medicine, Oncology. [Google Scholar]
- 18.Bejanyan N, Oran B, Shanley R, et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transplant. 2014;49(8):1029–35. doi: 10.1038/bmt.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Wang J. Precision therapy for acute myeloid leukemia. J Hematol Oncol . 2018;11(1):3. doi: 10.1186/s13045-017-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowe JM. Optimal induction and post-remission therapy for AML in first remission. Hematology Am Soc Hematol Educ Program. 2009:396–405. doi: 10.1182/asheducation-2009.1.396. [DOI] [PubMed] [Google Scholar]
- 21.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol . 2012;9(10):579–90. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- 22.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–61. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National Cancer Institute's Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biology of blood and marrow transplantation. Biol Blood Marrow Transplant. 2014;20(1):4–13. doi: 10.1016/j.bbmt.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alyea EP, DeAngelo DJ, Moldrem J, et al. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant . 2010;16(8):1037–69. doi: 10.1016/j.bbmt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stelljes M, Krug U, Beelen DW, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014;32(4):288–96. doi: 10.1200/JCO.2013.50.5768. [DOI] [PubMed] [Google Scholar]
- 26.Smith ML, Hills RK, Grimwade D. Independent prognostic variables in acute myeloid leukaemia. Blood Rev. 2011;25(1):39–51. doi: 10.1016/j.blre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–44. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 28.Fasslrinner F, Schetelig J, Burchert A, et al. Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol . 2018;5(4):e161–e169. doi: 10.1016/S2352-3026(18)30022-X. [DOI] [PubMed] [Google Scholar]
- 29.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 31.Mitus AJ, Miller KB, Schenkein DP, et al. Improved survival for patients with acute myelogenous leukemia. J Clin Oncol . 1995;13(3):560–9. doi: 10.1200/JCO.1995.13.3.560. [DOI] [PubMed] [Google Scholar]
- 32.Baron F, Labopin M, Niederwieser D, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26(12):2462–8. doi: 10.1038/leu.2012.135. [DOI] [PubMed] [Google Scholar]
- 33.Frazer J, Couban S, Doucette S, et al. Characteristics predicting outcomes of allogeneic stem-cell transplantation in relapsed acute myelogenous leukemia. Curr Oncol. 2017;24(2):e123–e130. doi: 10.3747/co.24.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbarzadeh Baghban A, hosseinifard H, Baghestani A R, et al. Factors that affecting survival of patients with acute myeloid leukemia. Koomesh. 2016;17(3):596–602. [Google Scholar]
- 35.Safar A, Rahgozar M, Shahei F, et al. Survival analysis of acute myeloid leukemia. RJMS. 2015;22(134):41–48. [Google Scholar]
- 36.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117(8):2307–18. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 37.Ganapule A, Nemani S, Korula A, et al. Allogeneic Stem Cell Transplant for Acute Myeloid Leukemia: Evolution of an Effective Strategy in India. J Glob Oncol. 2017;3(6):773–781. doi: 10.1200/JGO.2016.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michelis FV, Atenafu EG, Gupta V, et al. Duration of first remission, hematopoietic cell transplantation-specific comorbidity index and patient age predict survival of patients with AML transplanted in second CR. Bone Marrow Transplant. 2013;48(11):1450–5. doi: 10.1038/bmt.2013.71. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Tallman MS, Oken MM, et al. Duration of second complete remission compared with first complete remission in patients with acute myeloid leukemia. Eastern Cooperative Oncology Group. Leukemia. 2000;14(8):1345–8. doi: 10.1038/sj.leu.2401853. [DOI] [PubMed] [Google Scholar]
- 40.Sayehmiri K, Almasi E, Sarokhani D, et al. Prognostic factors for survival in acute leukemia patients after bone marrow transportation using Semi-Markov multi-state models in Tehran Shariati Hospital. Research in Medicine. 2013;36(5):83–7. [Google Scholar]
- 41.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant . 2015;21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jonge HJ, Valk PJ, de Bont ES, Schuringa JJ, Ossenkoppele G, Vellenga E, et al. Prognostic impact of white blood cell count in intermediate risk acute myeloid leukemia: relevance of mutated NPM1 and FLT3-ITD. Haematologica. 2011;96(9):1310–7. doi: 10.3324/haematol.2011.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padilha SL, Souza EJ, Matos MC, et al. Acute myeloid leukemia: survival analysis of patients at a university hospital of Parana. Rev Bras Hematol Hemoter. 2015;37(1):21–7. doi: 10.1016/j.bjhh.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su L, Li W, Cui JW, et al. [Correlation of NPM1, FLT3-ITD mutations with leukocyte count and myeloblasts percentage in AML patients with normal karyotype] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21(3):571–5. doi: 10.7534/j.issn.1009-2137.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 45.van Buchem MA, te Velde J, Willemze R, et al. Leucostasis, an underestimated cause of death in leukaemia. Blut. 1988;56(1):39–44. doi: 10.1007/BF00321058. [DOI] [PubMed] [Google Scholar]
- 46.Mousavinasab SN, Yazdani Cherati J, Karami H, et al. Risk Factors Influencing the Survival of Pediatric Acute Leukemia Using Competing Risk Model. J Mazandaran Univ Med Sci. 2015;24(121):31–8. [Google Scholar]
- 47.Krauter J, Wagner K, Stadler M, et al. Prognostic factors in allo-SCT of elderly patients with AML. Bone Marrow Transplant. 2011;46(4):545–51. doi: 10.1038/bmt.2010.145. [DOI] [PubMed] [Google Scholar]
- 48.Lipof JJ, Loh KP, O'Dwyer K, et al. Allogeneic Hematopoietic Cell Transplantation for Older Adults with Acute Myeloid Leukemia. Cancers (Basel) 2018;10(6):179. doi: 10.3390/cancers10060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hecker J, Miller I, Gotze KS, Verbeek M. Bridging Strategies to Allogeneic Transplant for Older AML Patients. Cancers (Basel) . 2018;10(7):232. doi: 10.3390/cancers10070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shokouhi S, Bray S, Bakhtiyari S, et al. Effects of aGVHD and cGVHD on Survival Rate in Patients with Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation. Int J Hematol Oncol Stem Cell Res. 2015;9(3):112–21. [PMC free article] [PubMed] [Google Scholar]
- 51.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–24. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1–7. doi: 10.1016/j.lrr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119(6):1599–606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 54.Sayemiri K, Eshraghian M, Mohammad K, et al. Predictive Factors of Survival Time after Hematopoietic Stem Cell Transplant in Acute Myeloid Leukemia Patients who Received Allogeneic BMT from Matched Sibling Donors Using Generalized Gamma Models. Int J Hematol Oncol Stem Cell Res. 2009;3(1):21–26. [Google Scholar]