FIGURE 6.
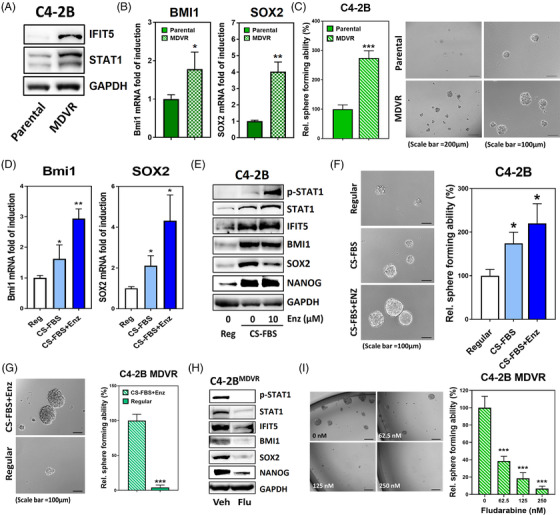
STAT1 signalling activation confers CSC properties in castration‐resistance PCa. (A) Elevation of STAT1 and IFIT5 protein in C4‐2B MDVR cells, compared to C4‐2B parental line. (B) Expression level of BMI1 and SOX2 mRNA in C4‐2B MDVR cells, compared to C4‐2B parental line. (C) Higher sphere forming ability of C4‐2B MDVR cells than C4‐2B parental cells. C4‐2B parental cells were cultured in 10% FBS RPMI‐1640 medium and C4‐2B MDVR cells were cultured in the Phenol Red free RPMI‐1640 medium supplemented with 10% CS‐FBS with additional 20 μM ENZ. (D) Induction of BMI1 and SOX2 gene upregulation in C4‐2B cells cultured in CS‐FBS‐supplemented phenol red‐free RPMI without (CS‐FBS) or with 10 μM ENZ (CS‐FBS+Enz), compared to regular culture condition (Reg). (E) Expression level of phosphorylated STAT1, STAT1, IFIT5, BMI1, SOX2 and NANOG proteins in C4‐2B cells cultured in CS‐FBS‐supplemented phenol red‐free RPMI without (CS‐FBS) or with 10 μM ENZ (CS‐FBS+Enz), compared to regular culture condition (Reg). (F) The sphere forming ability of C4‐2B cells primarily cultured in CS‐FBS‐supplemented pheno red‐free RPMI without (CS‐FBS) or with 10 μM ENZ (CS‐FBS+Enz) for 2 weeks, compared to regular culture condition (Reg). (G) The sphere forming ability of C4‐2B MDVR cells primarily cultured in regular condition for 2 weeks, compared with culture condition of CS‐FBS‐supplemented phenol red‐free RPMI with 20 μM ENZ (CS‐FBS+Enz). (H) The expression level of phosphorylated STAT1, STAT1, IFIT5, Bmi1, Sox2 and Nanog proteins in C4‐2B MDVR cells treated with fludarabine (500 nM, 48 h). (I) The sphere forming ability of C4‐2B MDVR cells treated with increased dose of fludarabine during sphere culture. C4‐2B MDVR cells were treated with fludarabine at corresponding concentration right after seeding into the ultra‐low attachment plate. Quantitation of spheres is done at 2 weeks after seeding. (*p < .05, **p < .001, ***p < .0001)
