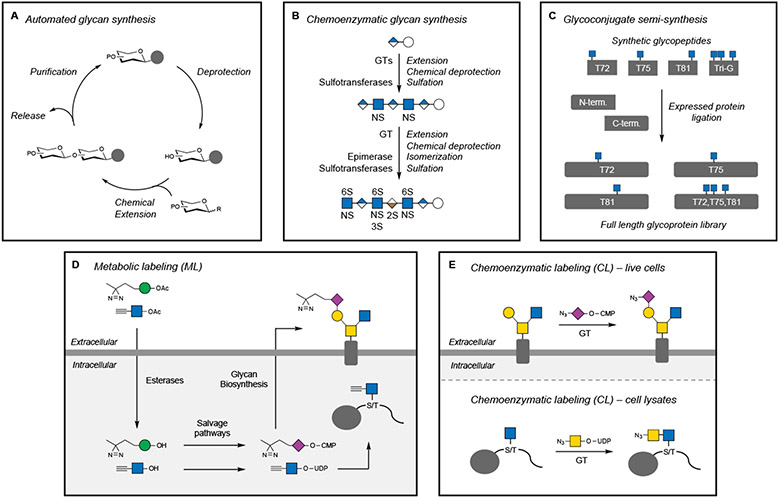
Figure 5. Methods to generate and modify glycans and glycoconjugates.
(A) Automated methods for the chemical synthesis of oligosaccharides use a similar approach as solid-phase peptide or oligonucleotide synthesis. The glycan is elongated through a series of deprotection and coupling steps, followed by release from the resin.
(B) The chemoenzymatic synthesis of an Arixtra biosimilar oligosaccharide employs multiple enzymatic and chemical steps to assemble and functionalize an HS heptasaccharide.
(C) Semi-synthesis of O-GlcNAcylated α-synuclein utilizes synthetic glycopeptide fragments for expressed protein ligation to generate a library of specific protein glycoforms.
(D) Metabolic labeling (ML) utilizes peracetylated (indicated by OAc) nonnatural monosaccharides that cross cell membranes, are deprotected (indicated by OH) by endogenous esterases, converted into nucleotide sugar donors, and then incorporated by GTs into glyconjugates. Nonnatural glycans with diazirine or alkyne functionalities are shown as representative examples.
(E) Chemoenzymatic labeling (CL) employs exogenous GTs and nonnatural nucleotide sugar donors to modify specific glycan structures recognized by the GT. Nonnatural glycans with azide functionalities are shown.