Abstract
Stenochlaena palustris fronds are popular as a vegetable in Southeast Asia. The objectives of this study were to evaluate the anticholinesterase properties and phytochemical profiles of the young and mature fronds of this plant. Both types of fronds were found to have selective inhibitory effect against butyrylcholinesterase compared with acetylcholinesterase. However, different sets of compounds were responsible for their activity. In young fronds, an antibutyrylcholinesterase effect was observed in the hexane extract, which was comprised of a variety of aliphatic hydrocarbons, fatty acids, and phytosterols. In the mature fronds, inhibitory activity was observed in the methanol extract, which contained a series of kaempferol glycosides. Our results provided novel information concerning the ability of S. palustris to inhibit cholinesterase and its phytochemical profile. Further research to investigate the potential use of this plant against Alzheimer’s disease is warranted, however, young and mature fronds should be distinguished due to their phytochemical differences.
Keywords: anticholinesterase, Stenochlaena palustris, Blechnaceae, phytochemical profile, frond maturity
1. Introduction
The central cholinergic system plays a key role in the regulation of cognitive functions. Damage in such systems is thought to be responsible for cognitive decline, which is the key features of Alzheimer’s disease (AD), dementia, and other neurodegenerative diseases [1]. Since cholinergic markers have been found to be greatly reduced in the postmortem brain samples of AD patients, and the decline in neurotransmitter acetylcholine can be correlated to the degree of cognitive impairment [2], inhibition of cholinesterases involved in the hydrolysis of acetylcholine is thought to be a plausible strategy for the treatment and control of memory loss associated with AD [3]. A number of foods from plants have been found to exhibit anticholinesterase activity. These include ginger [4], a blend of black chokeberry, and lemon juice [5], as well as green tea [6]. Other plant species containing alkaloids, xanthones, and polyphenols [7–9] have also been reported to exert inhibitory effects on cholinesterase and, thus, have potential applications in prevention of cognitive decline.
In this study, we evaluated the potential of a vegetable fern, Stenochlaena palustris, to inhibit cholinesterases and the phytochemicals involved. S. palustris is known as ‘Paku Miding’ in Malaysia, or ‘Kalakai” in Borneo and Kalimantan. It is a creeping fern found widely across India, through Malaysia and Polynesia, and into Australia [10]. The S. palustris frond is dimorphic and can be classified as fertile or sterile based on morphology. Fertile fronds have thin, long pinnae that bear spores, and are seasonal and inedible. However, the sterile fronds that are edible have broad pinnae with sharply toothed margins and are available throughout the year [11]. The young, sterile fronds of the fern have a crispy texture and are usually cooked with shrimp paste into a vegetable dish. They are also used traditionally to treat fever, diarrhea, skin diseases, cutaneous disorders, and gastric ulcers [11–13]. According to the Malaysian Agricultural and Development Institute, S. palustris has great potential to be exported to foreign markets. Therefore, research has been carried out to improve postharvest handling and packaging conditions in order to extend the storage period of the plant [14]. Furthermore, effort has also been undertaken to evaluate its cultivation and economic potential [15]. Despite having great promise in the food market, information concerning its health functions and nutritive properties remained scarce.
There have been accounts concerning the antifungal activity of the methanolic leaf extract [16] and antibacterial properties of flavonol glycosides [17] from S. palustris. Additionally, other researchers, including our group, found strong antioxidant activities and high polyphenolic content in the fronds [18–20]. Given that natural antioxidants may have potential in treating AD due to their neuroprotective properties [21,22], we explored the neuroprotective potential of S. palustris by evaluating its cholinesterase inhibitory properties. This study was carried out on young, sterile fronds, which are commonly consumed as vegetables, as well as on the mature sterile fronds, which are not usually eaten, in order to verify differences between them.
2. Materials and methods
2.1. Plant material
S. palustris (Burm.). Bedd. (Blechnaceae) was collected from Sungai Petani, Kedah, Malaysia. The taxonomic identity of the plant was authenticated by Ms Maliga Gnasan, a botanist at the Penang Botanic Gardens. A voucher specimen (No. 1645) was then deposited at the premises.
2.2. Chemicals and reagents
Hexane, dichloromethane (DCM), and methanol (MeOH) used for the extraction of plant materials were of analytical grade (Merck, Darmstadt, Germany). Reagents and standards used for estimating the total phenolic content and total flavonoid assays were: Folin-ciocalteu and quercetin hydrate from Sigma-Aldrich (St. Louis, MO, USA); sodium carbonate, sodium hydroxide, and sodium nitrite from Classic Chemicals (Shah Alam, Malaysia); gallic acid and aluminum chloride from Merck. For the anticholinesterase assay, acetylthiocholine iodide and acetylcholinesterase from electric eel, bovine serum albumin, 5,5′-dithiobis (2-nitrobenzoic acid), and butyrylcholinesterase from equine serum, and S-butyrylthiocholine chloride and physostigmine were purchased from Sigma-Aldrich.
2.3. Extraction of plant material
Prior to carrying out the extraction, young and mature fronds were separated based on their physical appearance. The young fronds are tender, juicy, and have a reddish–orange hue, while mature fronds are stiff and pure light green in color (Fig. S1). Upon separation, the fronds were cleaned, freeze-dried, and subsequently pulverized with a mill grinder. The powdered materials were extracted sequentially with n-hexane, DCM, and MeOH, twice for each solvent and for 20 minutes for each extraction, in a B-5510 ultrasonic cleaning bath operating at 42 kHz and 135 kW (Branson Ultrasonics Corporation, Danbury, CT, USA). The extracts obtained for each solvent were combined, filtered, and evaporated to dryness under reduced pressure at temperature < 40°C. The percentage yield of each plant extract was calculated based on the weight of the dried extract obtained (g) for every g of dried plant material used.
2.4. Cholinesterase inhibitory assay
Cholinesterase inhibitory potential of the samples was determined using a spectrophotometric method that was modified from that described by Ellman et al [23]. For the acetylcholinesterase (AChE) inhibition assay, 140 μL 0.1M Na2PO4 buffer (pH 8) was added to a 96-well microplate, followed by the addition of 20 μL test sample and 20 μL acetylcholinesterase enzyme (0.09 U/mL). Ten microliters 10mM 5,5′-dithiobis (2-nitrobenzoic acid) was then added to each well, followed by addition of 10 μL acetylthiocholine iodide (14mM). The absorbance of the colored end-product was measured at 412 nm at designated intervals for 30 minutes after initiation of the enzymatic reaction by a Infinite 200 ProMicroplate Spectrometer (Tecan, Männedorf, Switzerland).
For the butyrylcholinesterase (BChE) inhibition assay, the same procedure as described for AChE was followed; however, the enzymes and substrates were substituted with butyrylcholinesterase from equine serum and S-butyrylthiocholine chloride, respectively.
A set of five concentrations of each plant extract or isolated compound was used to estimate the 50% inhibitory concentration (IC50). Physostigmine, a cholinesterase inhibitor, was used as the reference standard. Absorbance of the test samples was corrected by subtracting the absorbance of their respective blank. Percentage inhibition was calculated using the following formula:
2.5. Phytochemical investigation
2.5.1. Gas chromatography-mass spectrometry analysis
Gas chromatography-mass spectrometry analysis (GC-MS) analyses of the hexane extract of both young and mature fronds was carried out on an Agilent 6890N Network GC system coupled to an Agilent 5973i Mass Selective Detector (Agilent Technologies, Waldbronn, Germany). Separation of the chemical compounds was achieved on an HP-5MS column (30 m × 0.25 mm, 0.25 μm; Agilent Technologies) with helium as the carrier gas flowing at 1.2 mL/min. The injection volume was 10 μL in a splitless mode. Initial temperature of the oven was 70° C for 2 minutes, which was increased to 280°C at the rate of 20°C/min. The column temperature was then maintained at 280°C for 20 minutes. The temperature of the injector was set at 280°C, while the temperature of the detector was 250°C. Mass acquisition was performed in the range of 40–550 a.m.u. using electron-impact ionization at 70 eV. Identification of the chemical components was done by performing spectral match against the National Institute of Standards and Technology database (Gaithersburg, MD, USA) and the Wiley Registry (John Wiley and Sons, Hoboken, NJ, USA). Similarity between the MS spectrum of the compounds and those in the database was evaluated by comparing the mass of their molecular ions, base ions, fragment ions, as well as their peak intensities. Only those compounds with >90% spectral matching quality were considered acceptable.
2.5.2. Total phenolic content
Total phenolic content (TPC) in the MeOH extract of S. palustris young and mature fronds was determined according to the method reported by Singleston and Rossi [24], with minor modifications. Briefly, 150 μL of each extract (1 mg/mL in MeOH) was diluted with 2.4 mL distilled water, followed by addition of 1 mL 0.2N Folin-Ciocalteu reagent, after which the solution was allowed to react for 5 minutes. Then, 300 μL of saturated Na2CO3 solution was added, and the mixture was allowed to incubate for 2 hours in the dark. An aliquot of 200 μL of the mixture was then transferred to a 96-well plate and the optical density was recorded at 725 nm using a microplate spectrophotometer (Multiskan Go; Thermo Scientific, Carlsbad, CA, USA). TPC of the samples was estimated from the calibration curve of gallic acid standard in the range of 0.01–0.1 mg/mL, and results were expressed as mg of gallic acid equivalents per gram of dry extract (mg GAE/g).
2.5.3. Total flavonoid content
Total flavonoid content (TFC) of the MeOH extracts was determined by the method developed by Sakanaka et al [25] with slight modifications. Briefly, 250 μL of plant extracts (1 mg/mL) were mixed with 1250 μL distilled water and 75 μL of 5% NaNO2 in a test tube. The mixture was incubated for 6 minutes, after which 150 μL of 10% AlCl3 solution was added to the mixture, and the reaction was allowed continue for 5 minutes. Then, 500 μL 1M NaOH was added and the mixture was brought to 2.5 mL with distilled water and stirred to mix well. An aliquot of 200 μL of the mixture was then transferred to a 96-well plate where the absorbance was measured immediately at 510 nm. TFC of the samples was determined from the calibration curve of the quercetin standard in the range of 0.01–0.1 mg/mL. Results were expressed in mg of quercetin equivalents per gram of dry extract (mg QE/g).
2.5.4. Phytochemical comparison by high-performance thin-layer chromatography
The phytochemicals present in the young fronds of S. palustris were qualitatively compared with that of the mature fronds by phytochemical comparison by high-performance thin-layer chromatography (HPTLC). Briefly, a solution of MeOH extract from each sample was prepared at 10 mg/mL in MeOH. A 10-μL aliquot was then spotted on a silica gel 60 HPTLC plate (Merck) and developed using a mobile-phase system that consisted of ethyl acetate (EtOAc), MeOH, and 1% acetic acid (8:1:1, v/v/v). The HPTLC plate was then treated with 1% AlCl3 and visualized at 365 nm. Yellow fluorescent bands were observed as an indication of the presence of flavonoids.
2.5.5. Flavonoid isolation and identification
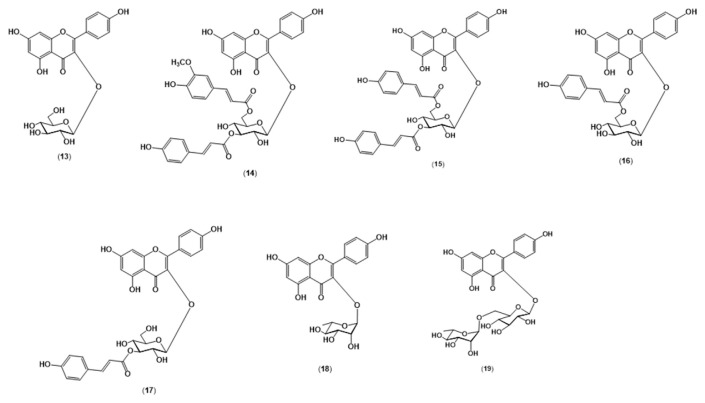
Dried MeOH extract from the mature fronds (50 g) was partitioned between EtOAc and water. The EtOAc-soluble portion was subjected to vacuum liquid chromatography on a silica gel (4.5 cm × 15.0 cm) using a step gradient of hexane-EtOAc-MeOH (5:5:0 to 0:0:10, v/v/v) to yield seven fractions (F1–F7). Purification of F1 was performed using silica gel flash-column chromatography (5.0 cm × 30 cm) at a flow rate of ~10 mL/min using a step gradient of hexane-CHCl3-MeOH (5:5:0 to 0:0:10, v/v/v; 15 mL fractions), followed by Sephadex LH-20 (2.5 cm × 30 cm, MeOH; 5 mL fractions; Sigma-Aldrich (St. Louis, MO, USA)), and subsequently prep-TLC with the developing solvent of CHCl3-MeOH (4.3: 0.7, v/v) to afford Compounds 14–18. Purification of F3 and F4 was achieved by Sephadex LH-20 (2.5 cm × 30 cm, MeOH; 5 mL fractions), followed by prep-TLC with a mobile phase system of EtOAc-MeOH-1% CH3COOH (8:1:1, v/v/v) to afford Compounds 13 and 19, respectively.
One- and two-dimensional nuclear magnetic resonance (NMR) spectroscopic data of the compounds was recorded on an Avance 500 NMR Spectrometer (Bruker, Vienna, Austria), while electrospray ionization-MS was obtained in negative mode on an AmaZon X mass spectrometer (Bruker). The isolated compounds were identified as kaempferol 3-O-β-glucopyranoside ([M-H]− = m/z 447.1; fragment ion m/z 284.8; Compound 13), kaempferol 3-O-(3″-O-E-p-coumaroyl)-(6″-O-E-feruloyl)-β-glucopyranoside ([M-H]− = m/z 769.3; fragment ions m/z 623.2, 593.2, 446.9, and 284.7; Compound 14), kaempferol 3-O-(3″,6″-di-O-E-p-coumaroyl)-β-glucopyrano-side ([M-H]− = m/z 739.3; fragment ions m/z 593.2, 446.9, and 284.7; Compound 15), kaempferol 3-O-(6″-O-E-p-coumaroyl)-β-glucopyranoside ([M-H]− = m/z 593.2; fragment ions m/z 446.9 and 284.7; Compound 16), kaempferol 3-O-(3″-O-E-p-coumaroyl)-β-glucopyranoside ([M-H]− = m/z 593.2; fragment ions m/z 446.9 and 284.7; Compound 17), kaempferol 3-O-α-rhamnopyranoside ([M-H]− = m/z 431.0; fragment ion m/z 284.7; Compound 18), and kaempferol 3-O-(6″-O-α-rhamnopyranoside)-β-glucopyranoside ([M-H]− = m/z 593.2; fragment ion m/z 284.6; Compound 19). NMR data for the compounds were in agreement with that reported in the literature [17,26–28].
2.6. Statistical analysis
Differences between the extraction yield of young and mature frond extracts was evaluated using Student t test at a 99.9% confidence interval. The relationship between TPC and TFC of the MeOH extracts were evaluated using Pearson’s correlation study. All statistical analyses were performed using SPSS software version 18.0 (SPSS, Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Total distribution of plant metabolites
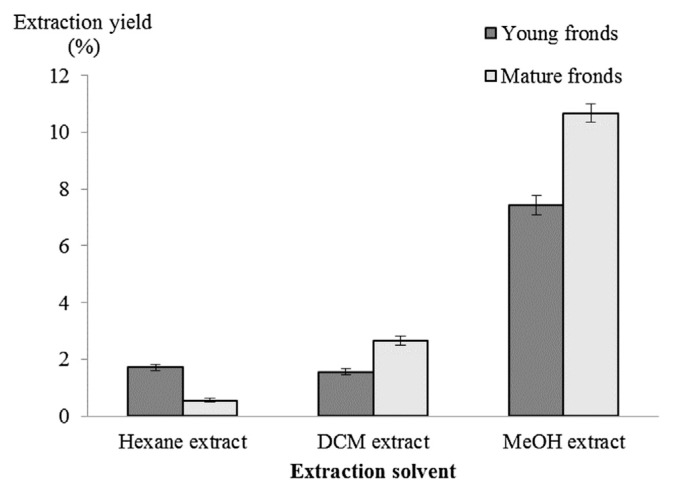
The total distribution of plant metabolites in the fronds of S. palustris at two different stages of maturity was compared by evaluating the extraction yield obtained using solvents of varying polarity. Among the hexane extracts, young fronds were found to produce higher extraction yields compared with the mature fronds, indicating that there are more lipophilic substances at the early stage of growth (Fig. 1). As the fronds mature, the content of lipophilic substances reduces, however, the production of hydrophilic compounds increases. This was shown by the higher extraction yield in both MeOH and DCM extracts in the mature fronds compared with the young fronds. These results indicated a change in biosynthesis and distribution of plant metabolites at different growth stages of S. palustris fronds.
Fig. 1.
Extraction yield of young and mature sterile fronds obtained using various solvents (results are mean ± SEM, n = 4). Significant differences (p < 0.01) were found between the extracts of young and mature fronds obtained with each solvent. SEM = standard error of the mean; DCM = dichloromethane; MeOH = methanol.
3.2. Cholinesterase inhibitory activity
Cholinesterase inhibitors have been shown clinically to be one of the most promising and preferred treatment for AD due to its efficacy and less severe side effects [29]. Two forms of cholinesterases that are the targets for inhibition are AChE and BChE, and in normal human brains, the activity of AChE predominates over BChE. As AD progresses, the activity of AChE declines in certain brain regions to 10–15% of normal activity, whereas BChE activity rises to partially compensate for the loss in AChE activity [30]. In terms of kinetic response, while AChE becomes substrate inhibited at high concentrations, BChE shows high efficiency in hydrolyzing acetylcholine at the corresponding concentration [31]. Hence, inhibition of both enzymes has complimentary implications in the treatment of mild-to-severe forms of AD.
In this study, the ability of S. palustris frond extracts to inhibit AChE and BChE were evaluated. The effects of various extracts from the young fronds toward cholinesterase enzymes were different compared with those of mature fronds (Table 1). The hexane extracts from young fronds showed prominent inhibitory effects against BChE, while the hexane extracts from the mature fronds exhibited 10-fold less inhibition of the same enzyme. By contrast, the MeOH extracts from the mature fronds showed strong activity against BChE and mild inhibitory effects against AChE, while the same type of extract from the young fronds exhibited no appreciable inhibition of both cholinesterases. DCM extracts from both young and mature fronds were inactive against the two enzymes. Our results indicated that S. palustris appeared to selectively inhibit BChE to a greater extent relative to AChE. This suggests that the plant may have potential applications in AD treatment at the moderate-to-advanced stages. In view of the vast differences in cholinesterase inhibitory activities between the extracts of young and the mature fronds, further analyses were carried out to compare phytochemical differences.
Table 1.
Cholinesterase inhibitory activity of Stenochlaena palustris.
| Sample | IC50 (μg/mL), mean ± SD | ||
|---|---|---|---|
|
| |||
| AChE | BChE | ||
| Hexane extract | Young fronds | >200 | 9.74 ± 0.33 |
| Mature fronds | >200 | 130.54 ± 13.46 | |
| DCM extract | Young fronds | >200 | >200 |
| Mature fronds | >200 | >200 | |
| MeOH extract | Young fronds | >200 | >200 |
| Mature fronds | 121.02 ± 10.04 | 19.77 ± 1.08 | |
| Physostigmine | 0.05 ± 0.01 | 0.16 ± 0.02 | |
AChE = acetylcholinesterase; BChE = butyrylcholinesterase; IC50 = concentration of a substance required to inhibit an enzymatic process by half; DCM = dichloromethane; SD = standard deviation.
3.3. Phytochemical comparison
3.3.1. Hexane extracts
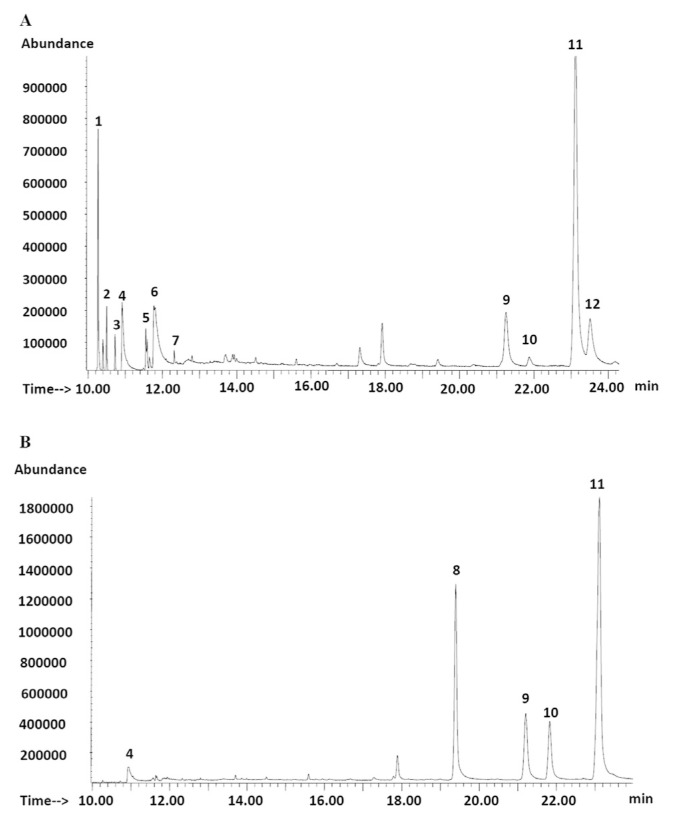
The hexane extracts from young and mature fronds were subjected to GC-MS analysis at a fixed concentration of 1 mg/mL. The MS data for the identified compounds are given in Table S1. Both samples were found to contain different varieties and concentrations of phytochemicals. The young fronds were rich in aliphatic compounds, essential fatty acids, and phytosterols. By contrast, the mature fronds contained relatively fewer constituents (Fig. 2). The main lipophilic components in the mature fronds were α-tocopherol and a number of phytosterols, while no aliphatic compounds or fatty acids were detected in the mature fronds, except for a trace amount of palmitic acid (Table 2). The presence of α-tocopherol and the phytosterols seemed to have little contribution to the cholinesterase activity of the extract, as the BChE inhibitory effect of the mature fronds was >10-fold weaker than that of the young fronds. Previous studies showed that β-sitosterol alone had no appreciable inhibitory effect on either AChE or BChE [32]. However, the presence of aliphatic hydrocarbons and fatty acids, as well as sterols, resulted in strong BChE inhibitory activity observed in the extracts from the young fronds, indicating the possibility of contributions by the aliphatic compounds toward anticholinesterase activity. Further work is required in order to confirm this finding. It should be noted that the variety of chemical constituents found in the hexane extract from the two fronds corresponded well with the extraction yield of the extracts. Greater constituent variety was observed in the extracts from the young fronds, which were obtained at higher yields compared with the fewer constituents obtained in the extracts from the mature fronds, which were obtained at lower yields.
Fig. 2.
A representative GC-MS chromatogram (TIC) of (A) young and (B) mature fronds of Stenochlaena palustris. The identity of the compounds: 1 = neophytadiene; 2 = transphytol; 3 = methyl palmitate; 4 = palmitic acid; 5 = methyl linoleate; 6 = linoleic acid; 7 = ethyl arachidonate; 8 = α-tocopherol; 9 = campesterol; 10 = stigmasterol; 11 = β-sitosterol; and 12 = fucosterol. GC-MS = gas chromatography mass spectrometry; TIC = total ion chromatogram.
Table 2.
Relative amount of chemical constituents in young and mature fronds of Stenochlaena palustris.
| Peak labels | Retention time (min) | Compound | Peak area (%)a | |
|---|---|---|---|---|
|
| ||||
| Young fronds | Mature fronds | |||
| 1 | 10.27 | Neophytadiene | 43.33 | — |
| 2 | 10.50 | trans-phytol | 12.22 | — |
| 3 | 10.73 | Methyl palmitate | 8.33 | — |
| 4 | 10.93 | Palmitic acid | 13.89 | 15 |
| 5 | 11.56 | Methyl linoleate | 8.33 | — |
| 6 | 11.79 | Linoleic acid | 12.22 | — |
| 7 | 12.33 | Ethyl arachidonate | 4.44 | — |
| 8 | 19.40 | α-tocopherol | — | 72.22 |
| 9 | 21.25 | Campesterol | 10 | 22.22 |
| 10 | 21.88 | Stigmasterol | 2.78 | 22.22 |
| 11 | 23.13 | β-sitosterol | 57.78 | 100 |
| 12 | 23.52 | Fucosterol | 10 | — |
Percentage of peak area relative to the largest peak, β-sitosterol, which is set at 100%.
Two components that were found to be mutually exclusive to each type of frond were fucosterol and α-tocopherol (Vitamin E). Fucosterol was found only in the hexane extract from the young fronds, while α-tocopherol was present only in the hexane extract from the mature fronds. Fucosterol is a precursor of β-sitosterol in the phytosterol biosynthesis pathway [33]. Therefore, the presence of fucosterol in young fronds suggests that the compound is not fully converted to β-sitosterol at the early stage of frond development. Fucosterol had been reported to possess weak BChE inhibitory activity [34], indicating that the compound may also partially contribute to overall anti-BChE activity observed in extracts from the young frond. α-Tocopherol was found in great abundance in the mature fronds. Although this compound has no direct effect on the anticholinesterase activities of the fern, it was reported to exert protective effects against neuronal cell death caused by oxidative stress [35]. These results suggested that the hexane extracts from both young and mature fronds of S. palustris play independent roles in neuroprotection.
3.3.2. Methanol extracts
To compare the phytochemicals between the MeOH extracts from young and mature fronds, TPC and TFC were first evaluated. As presented in Table 3, both young and mature fronds were found to have high content of polyphenols and flavonoids, in agreement with the findings of Chai et al [18]. However, TPC and TFC were approximately twofold higher in the mature fronds relative to the young fronds. The amount of phenolic substances present in 1 g of MeOH extract from the mature fronds was equivalent to ~1/4 g of gallic acid activity, while in the young fronds, the amount was only slightly >1/10 g gallic acid activity/g of extract. The flavonoid content in 1 g of the extract from mature fronds was equivalent to ~1/2 g of quercetin activity, while the same amount of extract from young fronds contain flavonoids equivalent to 1/5 g of quercetin activity. The high TPC values were found to be strongly correlated to their flavonoid content, as statistical analyses for the MeOH extract of both young and mature fronds showed r2 > 0.9 (Table 3).
Table 3.
Total phenolic and flavonoid content of the methanol extract from young and mature fronds of Stenochlaena palustris.
| Sample | Total phenolic content (mg GAE/g extract) | Total flavonoid content (mg QE/g extract) | Coefficient of determination (r2) |
|---|---|---|---|
| Young fronds | 94.15 ± 4.64 | 205.66 ± 0.07 | 0.912 |
| Mature fronds | 252.32 ± 13.27 | 503.56 ± 35.54 | 0.988 |
GAE = gallic acid equivalent; QE = quercetin equivalent.
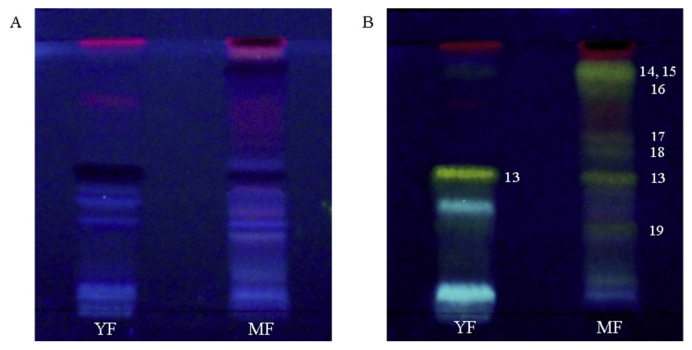
To further understand the differences observed in TPC and TFC values between the young and mature fronds, chemical constituents of both types of frond extracts were evaluated by HPTLC. Following postchromatographic treatment with AlCl3, flavonoid constituents were observed (Fig. 3). Based on our analysis, mature fronds contained a greater variety of flavonoids compared with the young fronds. The identity of these flavonoids were further determined by various spectroscopic techniques following isolation of the compounds and were found to be a series of kaempferol glycosides (Fig. 4). Among these substances, only kaempferol 3-O-β-glucopyranoside (Compound 13) was common to both young and mature fronds. The other flavonoids, which are derivatives of Compound 13, were found exclusively in the mature fronds. This indicates that, as the fronds mature, S. palustris tends to synthesize more complex molecules from its principal flavonoid, kaempferol 3-O-β-glucopyranoside (Compound 13). Large varieties and concentrations of such complex flavonoid molecules were likely to be the major contributors to the high TPC and TFC values observed in the MeOH extracts from mature fronds. The results were also consistent with the higher extraction yields observed in the MeOH extracts from mature fronds compared with the young fronds as discussed earlier in Section 3.1. Interestingly, the high anticholinesterase activity observed in the MeOH extract from mature fronds also corresponded well with its high content of flavonoids, suggesting a possible contribution of the kaempferol glycosides to the cholinesterase inhibitory effect outlined in Table 1. This is supported by a study reporting the effect of a variety of flavonols and their glycosides as cholinesterase inhibitors [36]. One of the compounds identified in this study, kaempferol 3-O-(6″-O-E-p-coumaroyl)-β-glucopyranoside (Compound 16), was also reported to have strong inhibitory effects on AChE, while the inhibitory effects on BChE have not yet been evaluated [37].
Fig. 3.
HPTLC of the MeOH extract from young fronds (YF) and mature fronds (MF). (A) Before and (B) after treatment with AlCl3 reagent. Flavonoids turned from dark to yellow fluorescent bands after treatment with AlCl3. The identity of the compounds are: kaempferol 3-O-β-glucopyranoside (Compound 13), kaempferol 3-O-(3″-O-E-p-coumaroyl)-(6″-O-E-feruloyl)-β-glucopyranoside (Compound 14), kaempferol 3-O-(3″,6″-di-O-E-p-coumaroyl)-β-glucopyranoside (Compound 15), kaempferol 3-O-(6″-O-E-p-coumaroyl)-β-glucopyranoside (Compound 16, kaempferol 3-O-(3″-O-E-p-coumaroyl)-β-glucopyranoside (Compound 17), kaempferol 3-O-α-rhamnopyranoside (Compound 18), and kaempferol 3-O-(6″-O-α-rhamnopyranoside)-β-glucopyranoside (Compound 19). HPTLC = high-performance thin-layer chromatography; MeOH = methanol.
Fig. 4.
Kaempferol glycosides isolated from the mature sterile fronds of Stenochlaena palustris. The identity of the compounds: kaempferol 3-O-β-glucopyranoside (Compound 13), kaempferol 3-O-(3″-O-E-p-coumaroyl)-(6″-O-E-feruloyl)-β-glucopyranoside (Compound 14), kaempferol 3-O-(3″,6″-di-O-E-p-coumaroyl)-β-glucopyranoside (Compound 15), kaempferol 3-O-(6″-O-E-p-coumaroyl)-β-glucopyranoside (Compound 16), kaempferol 3-O-(3″-O-E-p-coumaroyl)-β-glucopyranoside (Compound 17), kaempferol 3-O-α-rhamnopyranoside (Compound 18), and kaempferol 3-O-(6″-O-α-rhamnopyranoside)-β-glucopyranoside (Compound 19).
To verify whether the isolated kaempferol glycosides (Compounds 13–19) contributed to the anticholinesterase activity of the MeOH extracts, the individual compounds were evaluated for their ability to inhibit BChE. Of the seven compounds, two diacylated kaempferol glycosides, kaempferol 3-O-(3″-O-E-p-coumaroyl)-(6″-O-E-feruloyl)-β-glucopyranoside (Compound 14) and kaempferol 3-O-(3″,6″-di-O-E-p-coumaroyl)-β-glucopyranoside (Compound 15), showed moderate inhibitory effects on BChE, with IC50 values of 87.44 μg/mL and 63.12 μg/mL, respectively (Table 4). The rest of the constituents appeared to have very mild inhibitory activities on the enzyme at the test concentration of 100 μg/mL. Based on the structure of the compounds, acylation of the kaempferol glycosides with hydroxycinnamic acids on both positions 3″ and 6″ of the glucose unit seemed to contribute greatly to the inhibitory effects of the kaempferol glycosides on BChE. However, by comparing the activity of Compound 14 to Compound 15, the former had a ferulyol moiety at position 6″ and demonstrated a lower inhibitory potential against BChE compared with the latter, which had a p-coumaroyl moiety at the same position. This suggested that substitution of a meta-hydrogen with a methoxy group on the hydroxycinnamyol unit reduced the activity of the compound. Nevertheless, the weaker anti-BChE activity of the individual compounds as compared to the MeOH extracts from which they were isolated indicated that no single compound may be solely responsible for the activity of the extract. The prominent anticholinesterase activity of the MeOH extracts from mature fronds may be due to a complex synergism between the flavonoids and other constituents that were not determined in this study.
Table 4.
BChE inhibitory activity of kaempferol glycosides of Stenochlaena palustris fronds.
| Compound | % Inhibition at 100 μg/mLa | IC50, μg/mL (μM)a |
|---|---|---|
| 13 | 7.45 ± 0.72 | ND |
| 14 | 54.18 ± 2.93 | 87.44 ± 5.86 (113.66 ± 7.66) |
| 15 | 52.10 ± 1.11 | 63.12 ± 6.12 (85.37 ± 7.62) |
| 16 | 16.82 ± 2.18 | ND |
| 17 | 6.90 ± 0.67 | ND |
| 18 | 9.45 ± 1.17 | ND |
| 19 | 0 | ND |
| Physostigmine | 94.94 ± 0.04 | 0.16 ± 0.02 (0.59 ± 0.07) |
BChE = butyrylcholinesterase; IC50 = concentration of a substance required to inhibit an enzymatic process by half; ND = not determined; SD = standard deviation.
Data presented as mean ± SD (n = 3).
The fate of the acylated kaempferol glycosides isolated from S. palustris when entering the in vivo system remains uncertain. However, it can be assumed that these molecules will largely undergo hydrolysis when ingested to form two basic units, consisting of kaempferol aglycone and hydroxycinnamic acid, through cleavage of the glycosidic bonds by enteric enzymes and colon microflora [38]. These liberated molecules are reported to have better absorption efficacy than pure kaempferol [39,40]. Numerous studies showed that free kaempferol and hydroxycinnamic acids were potent natural cholinesterase inhibitors, with this a key target in AD treatment [36,41,42]. Behavioral studies on mice with valium-impaired memory and using a radial arm maze model showed that kaempferol administration significantly improved the spatial learning and memory of the mice [43]. Additionally, simple hydroxycinnamic acids, such as p-coumaric and ferulic acid, were reported to attenuate β-amyloid-induced neurotoxicity in mice by binding to acetycholine and destabilizing the structure of the growing β-amyloid fibrils [42]. Although the acylated glycosides may be hydrolyzed in vivo, they could still play a role as precursors for pharmacologically active kaempferol and hydroxycinnamic acids. Nevertheless, if the acylated kaempferol glycosides are to be taken with other S. palustris constituents in the form of an extract, in vivo transformation of these compounds may vary, as the presence of multiple components in plants may help stabilize one another against enzymatic degradation and enhance their solubility in water [44]. An example of a plant extract containing acylated flavonol glycosides and that has been experimentally and clinically shown to increase cognitive functions is the standardized Gingko biloba leaf extract, EGb 761 [45]. Future studies should concentrate on the pharmacodynamics and pharmacokinetics of S. palustris extracts and its individual kaempferol glycosides in order to further explore their potential contribution toward AD treatment.
4. Conclusion
This study demonstrated that S. palustris exhibits selective inhibitory effects on BChE. The distribution of phytochemicals in S. palustris fronds at different stages of maturity varies, thereby resulting in a great contrast in cholinesterase inhibitory activity from the same type of extracts. Extracts from young fronds contain a mixture of aliphatic substances, fatty acids, and phytosterols, while extracts from active mature fronds contain a variety of kaempferol glycosides. The individual kaempferol glycoside isolated from the plant was found to have weaker anti-BChE activity relative to the extract from which they were isolated. Based on these findings, the edible young fronds of S. palustris have potential as a functional food to prevent cognitive decline. The mature fronds should also be explored for their potential as a therapeutic for AD. It is critical to clearly define the stage of frond maturity in future S. palustris investigations due to the distinction between young and mature fronds in terms of chemistry, biology, and their potential contribution to human health.
Acknowledgements
This study was supported by Universiti Sains Malaysia under the Short-term Grant (304/CDADAH/6311104) and Research University Grant (1001/CDADAH/815096). NJYC is a recipient of the MyBrain scholarship funded by the Ministry of Education, Malaysia.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2015.12.005.
Funding Statement
This study was supported by Universiti Sains Malaysia under the Short-term Grant (304/CDADAH/6311104) and Research University Grant (1001/CDADAH/815096). NJYC is a recipient of the MyBrain scholarship funded by the Ministry of Education, Malaysia.
Footnotes
Conflicts of Interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 2. Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry R. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Brit Med J. 1978;2:1457–9. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mukherjeea PK, Venkatesan K, Mal K, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4. Okonogi S, Chaiyana W. Enhancement of anti-cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov Ther. 2012;6:249–55. [PubMed] [Google Scholar]
- 5. Gironés-Vilaplana R, Valentão P, Andrade PB, Ferreres F, Moreno DA, García-Viguera C. Phytochemical profile of a blend of black chokeberry and lemon juice with cholinesterase inhibitory effect and antioxidant potential. Food Chem. 2012;134:2090–6. doi: 10.1016/j.foodchem.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 6. Okello EJ, Leylabi R, McDougall GJ. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct. 2012;3:651–61. doi: 10.1039/c2fo10174b. [DOI] [PubMed] [Google Scholar]
- 7. Urbain A, Marston A, Grilo LS, Bravo J, Purev O, Purevsuren B, Batsuren D, Reist M, Carrupt PA, Hostettmann K. Xanthones from Gentianella amarella ssp. acuta with acetylcholinesterase and monoamine oxidase inhibitory activities. J Nat Prod. 2008;71:895–7. doi: 10.1021/np070690l. [DOI] [PubMed] [Google Scholar]
- 8. Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 2012;11:329–45. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9. Konrath EL, Santos-Passos C, Klein-Júnior LC, Henriques AT. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer’s disease. J Pharm Pharmacol. 2013;65:1701–25. doi: 10.1111/jphp.12090. [DOI] [PubMed] [Google Scholar]
- 10.Holttum RE. A revised flora of Malaya. Vol. 2. Singapore: Government Printing Office; 1968. [Google Scholar]
- 11.Piggott AG. Ferns of Malaysia in colour. Kuala Lumpur, Malaysia: Tropical Press; 1988. [Google Scholar]
- 12. Suhartono A, Suhartono E. Stenochlaena palustris aqueous extract reduces hepatic peroxidative stress in Marmota caligata with induced fever. Universa Medicina. 2010;29:123–8. [Google Scholar]
- 13. Benniamin A. Medicinal ferns of North Eastern India with special reference to Arunachal Pradesh. Indian J Tradit Know. 2011;10:516–22. [Google Scholar]
- 14.Nicholas D, Chua HP, Saniah K.Postharvest treatment and packaging system for Sarawak’s indigenous midin fern (Stenochlaena palustris). Proceedings of the second international symposium on underutilized plant species: crops for the future–beyond food security; Kuala Lumpur, Malaysia: ISHS; 2013. [Google Scholar]
- 15. Mertz O. Cultivation potential of two edible ferns, Diplazium esculentum and Stenochlaena palustris. Trop Agr. 1999;76:10–6. [Google Scholar]
- 16. Sumathy V, Lachumy JS, Zuraini Z, Sasidharan S. Effects of Stenochlaena palustris leaf extract on growth and morphogenesis of food borne pathogen Aspergillus niger. Malays J Nutr. 2010;16:439–46. [PubMed] [Google Scholar]
- 17. Liu H, Orjala J, Sticher O, Rali T. Acylated flavonol glycosides from leaves of Stenochlaena palustris. J Nat Prod. 1999;62:70–5. doi: 10.1021/np980179f. [DOI] [PubMed] [Google Scholar]
- 18. Chai TT, Panirchellvum E, Ong HC, Wong FC. Phenolic contents and antioxidant properties of Stenochlaena palustris, an edible medicinal fern. Bot Stud. 2012;53:439–46. [Google Scholar]
- 19. Suhartonoa E, Viani E, Rahmadhan MA, Gultom IS, Rakhman MF, Indrawardhana D. Total flavonoid and antioxidant activity of some selected medicinal plants in South Kalimantan of Indonesian. APCBEE Procedia. 2012;4:235–9. [Google Scholar]
- 20. Ponnusamy Y, Chear NJY, Ramanathan S, Murugaiyah V, Lai CS. Antioxidant and antibacterial properties of Malaysian ferns used traditionally against infection. J Nat Prod Plant Resour. 2013;3:14–8. [Google Scholar]
- 21. Cole GM, Morihara T, Lim GP, Yang F, Begum A, Frautschy SA. NSAID and antioxidant prevention of Alzheimer’s disease: lessons from in vitro and animal models. Ann NY Acad Sci. 2004;1035:68–84. doi: 10.1196/annals.1332.005. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Zhao B. Natural antioxidants in prevention and management of Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:794–808. doi: 10.2741/419. [DOI] [PubMed] [Google Scholar]
- 23. Ellman GL, Courtney D, Anddres KDV, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 24. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–58. [Google Scholar]
- 25. Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinohacha) Food Chem. 2005;89:569–75. [Google Scholar]
- 26. Song N, Xu W, Guan HF, Liu XQ, Wang YB, Nie XL. Several flavonoids from Capsella bursa-pastoris (L.) Medic. Asian J Tradit Medic. 2007;2:218–22. [Google Scholar]
- 27. Susanti D, Sirat HM, Ahmad F, Ali RM, Aimi N, Kitajima M. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chem. 2007;103:710–6. [Google Scholar]
- 28. Badria FA, Ameen M, Akl MR. Evaluation of cytotoxic compounds from Calligonum comosum L. growing in Egypt. Z Naturforsch. 2007;62:656–60. doi: 10.1515/znc-2007-9-1005. [DOI] [PubMed] [Google Scholar]
- 29. Fan LY, Chiu MJ. Combotherapy and current concepts as well as future strategies for the treatment of Alzheimer’s disease. Neuropsychiatr Dis Treat. 2014;10:439–51. doi: 10.2147/NDT.S45143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacobini E. Drugs that target cholinesterases. In: Bucxafusco JJ, editor. Cognitive enhancing drugs. Switzerland: Birkhäuser Verlag; 2004. pp. 11–36. [Google Scholar]
- 31. Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu QS, Mamczarz J, Holloway HW, Giordano T, Chen DM, Furukawa K, Sambamurti K, Brossi A, Lahiri DK. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer-amyloid peptide in rodent. Proc Natl Acad Sci U S A. 2005;102:17213–8. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kolaki U, Boğa M, Akalin-Uruşak E. Constituents of Plantago major subsp. intermedia with antioxidant and anticholinesterase capacities. Turk J Chem. 2011;35:637–45. [Google Scholar]
- 33. Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agr. 2000;80:939–66. [Google Scholar]
- 34. Yoon NY, Chung HY, Kim HR, Choi JS. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fisheries Sci. 2008;74:200–7. [Google Scholar]
- 35. Behl C. Vitamin E protects neurons against oxidative cell death in vitro more effectively than 17β-estradiol and induces the activity of the transcription factor NF-κB. J Neural Transm. 2000;107:393–407. doi: 10.1007/s007020070082. [DOI] [PubMed] [Google Scholar]
- 36. Katalinić M, Rusak G, Barovic JD, Sinko G, Jelic D, Antolovic R, Kovarik Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur J Med Chem. 2010;45:186–92. doi: 10.1016/j.ejmech.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 37. Jung M, Park M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules. 2007;12:2130–9. doi: 10.3390/12092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–84. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 39. Hollman PCH, Vries JHM, Leeuwen SD, Mengelers MJB, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276–82. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 40. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 41. Min BS, Cuong TD, Lee JS, Shin BS, Woo MH, Hung TM. Cholinesterase inhibitors from Cleistocalyx operculatus. Arch Pharm Res. 2010;33:1665–70. doi: 10.1007/s12272-010-1016-5. [DOI] [PubMed] [Google Scholar]
- 42. Szwajgier D, Borowiec K. Phenolic acids from malt are efficient acetycholinesterase and butyrylcholinesterase inhibitors. J Inst Brew. 2012;118:40–8. [Google Scholar]
- 43. Bahrani H, Mohamad J, Paydar MJ, Rotham HA. Isolation and characterization of acetylcholinesterase inhibitors from Aquilaria subintegra for the treatment of Alzheimer’s disease (AD) Curr Alzheimer Res. 2014;11:206–14. doi: 10.2174/1567205011666140130151344. [DOI] [PubMed] [Google Scholar]
- 44. Viskupičová J, Ondrejovič M, Šturdík E. The potential and practical applications of acylated flavonoids. Pharmazie. 2009;64:355–60. [PubMed] [Google Scholar]
- 45. Kehr J, Yoshitake S, Ijiri S, Koch E, Nöldner M, Yoshitake T. Ginkgo biloba leaf extract (EGb 761) and its specific acylated flavonol constituents increase dopamine and acetylcholine levels in the rat medial prefrontal cortex: possible implications for the cognitive enhancing properties of EGb 761. Int Psychogeriat. 2012;24:S25–34. doi: 10.1017/S1041610212000567. [DOI] [PubMed] [Google Scholar]