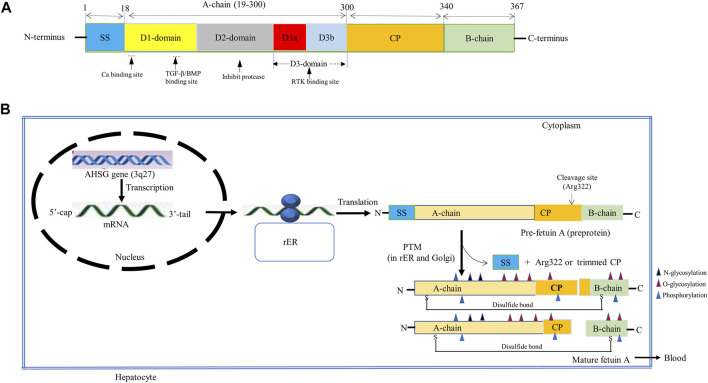
FIGURE 1.
Schematic representation of (A) Fetuin-A structure. The pre-fetuin-A contains 367 amino acids, including 18 amino acid size SS (Pedersen, 1944; Brown et al., 1992; Dziegielewska and Brown, 1995; Brown and Dziegielewska, 1997; Olivier et al., 2000; Denecke et al., 2003; Kettler et al., 2003; Stefan et al., 2006a; Häusler et al., 2009; Wojtysiak-Duma et al., 2010; Jahnen-Dechent et al., 2011; Mori et al., 2012; Dietzel et al., 2013; Cerdà‐Costa and Xavier Gomis‐Rüth, 2014; Stöcker et al., 2014; Floehr et al., 2016; Lin et al., 2018; Sardana et al., 2021); 282 amino acids A-chain (19–300), 40 amino acids CP (301–340), and 27 amino acids long B-chain (341–367). A-chain is composed of two cystatin-like domains (D1 and D2) and a variable non-cystatin domain, D3 (D3a and D3b). Functionally, the D1 provides binding sites for calcium, TGF- β, and BMP, the D2 inhibits cysteine protease and D3 interacts with the insulin receptor. (B) Fetuin-A biosynthesis. Fetuin-A is encoded by the AHSG gene located on the 3q27 locus that produces a single copy of mRNA encoding a pre-fetuin-A. The pre-fetuin-A consisting of SS, A-chain, CP, and A-chain undergoes PTMs (glycosylation, proteolysis, folding, and phosphorylation). Glycosylation takes place at Asp residues (N-glycosylation), Thr and Ser residues (O-glycosylation). Then SS and Arg322 will be removed through proteolysis with certain unknown proteinase to form a mature fetuin-A containing a full length of CP apart from Arg322 or C-terminally trimmed CP, interlinked by a disulfide bond formed between Cyst-14 and Cys-340. Then phosphorylation at multiple Ser and Thr residues, mostly in plasma, occurs by FAM20C. Abbreviations: AHSG, α2-Heremans-Schmid glycoprotein; BMP, Bone Morphogenic protein; CP, connecting peptide; FAM20C, family with sequence similarity 20 member C; PTMs, post-translational modifications; rER, rough endoplasmic reticulum; RTK, receptor tyrosine kinase; SS, signal sequence; TGF- β, Transforming growth factor-beta.