Abstract
Despite huge advances in understanding the molecular basis of IBD, clinical management has continued to rely on a “trial and error” approach. In addition, a therapeutic ceiling has emerged whereby even the most effective interventions are only beneficial for approximately 30% of patients. Consequently, several tools have been developed to aid stratification and guide treatment-decisions.
We review the potential application for many of these precision medicine approaches, which are now almost within reach. We highlight the importance of early action (and avoiding inaction) to ensure the best outcomes for patients and how combining early action with precision tools will likely ensure the right treatment is delivered at the right time and place for each individual person living with IBD.
The lack of clinical impact to date from precision medicine, despite much hype and investment, should be tempered with the knowledge that clinical translation can take a long time, and many promising breakthroughs might be ready for clinical implementation in the near future. We discuss some of the remaining challenges and barriers to overcome for clinical adoption. We also highlight that early recognition, early diagnosis, early stratification, and early intervention go hand in hand with precision medicine tools. It is the combination of these approaches that offer the greatest opportunity to finally deliver on the promise of precision medicine in IBD.
Keywords: precision medicine, stratified medicine, biomarkers, early diagnosis, early intervention
Introduction
There has been a rapid global rise in inflammatory bowel disease (IBD),1 and there are now well-recognized epidemiological phases that have contributed to the increasing prevalence of both ulcerative colitis (UC) and Crohn’s disease (CD).2 Although the incidence in the Western world seems to have plateaued,3 there remains ongoing rapid increases in incidence across many emerging nations, particularly lower and middle income countries (LMICs) with large populations.4 This rise has been mirrored across a range of other immune-mediated inflammatory diseases (IMIDs),5 leading many to conclude that much of this pattern is being driven by exposure to environmental risk factors. However, many of these environmental risk factors remain largely unknown or poorly understood.6
Paralleling the numbers of patients with IBD, there has been a steady increase in the number of treatment options available.7 Despite all this progress, management decisions are typically still made using a “one size fits all” approach. Given the historical lack of clinically useful predictors, patients have typically started a treatment with response and tolerability simply being assessed at clinical follow-up appointments. However, a number of recent advances have offered hope that the prospect of precision medicine tools may be ready for clinical implementation in the near future, and many people have focused on these tools as being critical to deliver precision medicine.8 We argue that as precision medicine involves delivering the best outcomes for each individual patient, simple measures such as earlier action (and avoiding inaction) are just as important as these more novel biomarkers and models. Based on expert consensus and experience, we summarize the most promising articles and findings to date and highlight how ongoing and remaining challenges could be overcome by using precision medicine tools early in a disease course and how this might help realize the aspiration of delivering precision medicine in IBD (Figure 1).
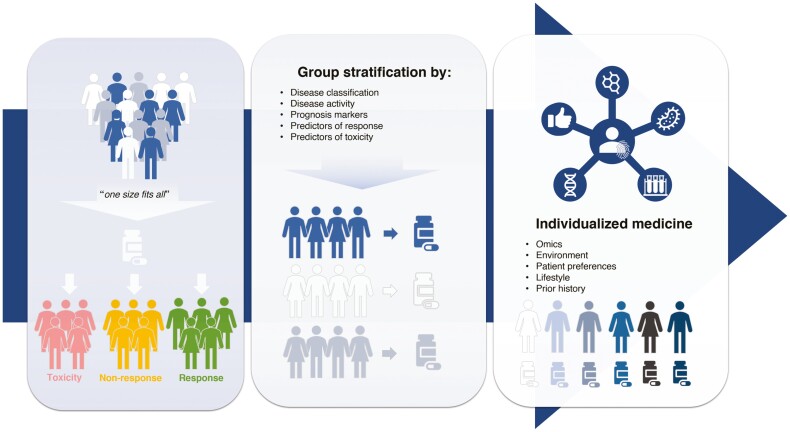
Figure 1.
Diagram to show how use of precision medicine tools and stratification should enable delivery of individualized medicine in IBD. Highlighting the importance of stratification to move away from a “one size fits all” approach towards an individualized medicine approach.
Early Diagnosis
It has consistently been demonstrated that there are significant delays in presentation, referral, and diagnosis for both UC and CD.9 Despite major improvements in many aspects of IBD clinical care over the last few decades, including a greater number of tools to help establish a diagnosis of either UC or CD, there appears to have been no major reduction in diagnostic delay for either condition.10 This diagnostic delay has been reported to be greater in CD,11 which is likely due to many factors including that patients can typically present with nonspecific symptoms such as abdominal pain, constipation, or diarrhea which may be mistaken for other gastrointestinal disorders, including irritable bowel syndrome.12 Delayed diagnosis has also been associated with more emergent presentation, which in turn has been associated with more adverse clinical outcomes.11, 13
The issue of delayed diagnosis is further compounded by an increasing awareness that many patients report prevalent symptoms for several years before a formal diagnosis of IBD is established.14 Unsurprisingly, in this period before diagnosis of IBD, high health care usage has been reported as patients seek help to manage their symptoms.15 In addition to symptoms being present, simple laboratory abnormalities are often detectable even up to 12 months before diagnosis in patients with CD.16 Accordingly, one solution to overcome these delays in diagnosis is likely to be through increasing awareness in the medical community and encouraging use of tools to identify red flags, which allow more timely referrals to specialist clinics and greater pickup for cases of early IBD.17,18
Taking this one step further and learning from analogous IMIDs such as rheumatoid arthritis and type 1 diabetes mellitus, there is an increasing understanding for the phenomenon of preclinical IBD.19 Indeed, much like other IMIDs, the initial study of preclinical IBD has focused on serum samples from a number of different cohorts that have been collected prior to development of disease.20 In this regard, the most important findings on preclinical IBD have been provided by the Genetic, Environmental, and Microbial (GEM) project and the Proteomic Evaluation and Discovery in an IBD Cohort of Tri-severe Subjects (PREDICTS) study. The GEM project recruited a cohort of patients who were already at higher risk of IBD, given a prior family history of this disease, and demonstrated that altered gut microbiota is associated with fecal proteolytic activity in patients prior to development of UC.21 However in CD, there appears to be an increase in intestinal permeability prior to the development of disease.22 Similarly, the PREDICTS cohort demonstrated that a panel of serum antibodies and proteins was able to predict the likelihood of patients developing CD within 5 years, with a high degree of accuracy.20 The findings from the GEM project and PREDICTS study are consistent and might suggest that in early disease development (at least for CD), there is increased intestinal permeability, allowing microbial antigens to be sampled by antigen-presenting cells and stimulating the production of antimicrobial antibodies and peptides.
Ongoing challenges remain for the study of preclinical IBD, given that the proteomic findings in the CD cohort from the PREDICTS study were not replicated in UC. Indeed, one of the limitations of preclinical IBD investigation to date has been the focus on single-omics platforms, and in this regard, results from the IBD-Character consortium should provide further key multi-omic insights for development of IBD.23An additional limitation that has not yet been overcome in the preclinical IBD field is that currently there are no clear-cut strategies for “disease interception” to prevent development of IBD.24 In addition to future consideration of medical therapeutics for disease interception, modulation of risk factors such as smoke exposure, antibiotic exposure, certain dietary foods, or identifying mechanisms of protective factors such as breastfeeding in early life may prove to be critical in the efforts for primary prevention of developing IBD.6 One area of growing awareness is how dietary patterns can drive pro-inflammatory and anti-inflammatory gut microbial ecosystems.25 Accordingly, we look forward to results from the PREdiCCt study (NCT03282903), which is prospectively examining over 2600 patients with IBD from the United Kingdom, to provide a greater understanding on the role of diet and microbiota as triggers for inflammation.
The subject of preclinical IBD highlights perfectly that although primary prevention and disease interception are very interesting and worthwhile aspects to focus on for future research, in the immediate-term there needs to be an emphasis on early recognition and diagnosis. Simple tools to aid this include the use of red flags for referrers, more timely referral to specialists, and early diagnosis; these will very likely contribute to improved outcomes for individual patients.
Early Stratification
The importance of early stratification has been highlighted and explored in detail recently.26 Although many clinical risk prediction models have been developed, the majority of these have failed to be validated in external cohorts or have lacked sufficient accuracy to help guide clinical decision-making.27 As a result, attention has turned towards biomarkers to aid stratification, with particular focus on biomarkers predictive of prognosis, response to therapeutics, and likelihood of developing side effects to therapeutics.28
Prognosis
Determining prognosis, or disease outcome, has increasingly been recognized as an important foundation for the delivery of precision medicine in IBD.26 However, currently clinicians are restricted to simple clinical features to help prognosticate for patients after a diagnosis has been established.29 Some of these clinical features clearly associate with worse prognosis such as complex, fistulizing peri-anal disease; however for the vast majority of patients, many clinical factors—typically identified from small, retrospective, observational cohorts—have lacked accuracy and not proved sufficiently helpful to guide therapeutic decision-making.30 Accordingly, most guidelines have continued to advise treating the majority of patients with IBD in a stepwise manner where patients are offered treatments in a “one size fits all” approach.31 This is despite the significant heterogeneity in disease course across IBD, with subgroups of individuals noted to have a milder, more quiescent disease course and other subgroups observed to have a more severe, treatment-refractory disease course.32, 33
Initially, there was great interest and enthusiasm for the potential use of circulating antibodies against bacterial antigens as prognostic markers34; however, serological antibodies are a good illustration of the need to distinguish between association and true prediction of worse outcomes.35,36 Antibodies of interest included anti-Saccharomyces cerevisiae (ASCA), perinuclear antineutrophil cytoplasmic (pANCA), antibody to CBir1flagellin (anti-CBir1), antibody to Escherichia coli outer membrane porin C (anti-OmpC), and others that had been associated with adverse outcomes in patients with CD.37 However, an important consideration is that many of these are the same antibodies associated with preclinical disease24 and likely represent patients with longstanding CD who have remained untreated and therefore associated with worse outcomes, rather than truly being predictive of prognosis.
Similarly, given the success of genome-wide association studies (GWASs) to demonstrate single-nucleotide polymorphisms (SNPs) associated with disease susceptibility, many in the field of genetics thought there may be similar success to better understand disease outcomes. Genome-wide association studies have suggested that there are distinct genes associated with a worse disease course in both CD38 and UC39 and that these genes are distinct to the ones implicated in disease susceptibility. However, due to the low odds ratios of these genetic associations, they have not proved useful to test as part of routine clinical practice. A potentially exciting future application may be that polygenic risk scores (PRSs) could be used to predict outcomes for patients with IBD40; however, this will first require much larger and much more diverse genome-wide population studies in order to become a reality for the future.41
Given the inability of GWASs to prognosticate in IBD, much attention has subsequently switched to the utility of gene expression, measured using transcriptomic technologies. Much of the initial focus was understandably on gene expression at a tissue level, given that this is the site of inflammation in IBD. The RISK cohort (Risk Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn’s Disease) of newly diagnosed pediatric CD has provided many insights in this regard, seeking to develop risk prediction models based on gene-expression data from colonic biopsies combined with microbial and clinical data.42 This study has also found an extracellular matrix signature when analyzing ileal biopsies that have been associated with greater development of stricturing disease within 5 years (AUC, 0.81).43 Work is currently ongoing to translate these findings to simple and clinically usable biomarkers, including assessment of blood-based biomarkers that would generally be more acceptable for patients compared with biopsy-based biomarkers requiring invasive, endoscopic procedures.44
The benefits of focusing on noninvasive blood-based biomarkers are well-illustrated by the translation of a CD8 T-cell signature, which is associated with worse outcomes in patients with newly diagnosed IBD.45 This CD8 T-cell signature was originally identified after cell separation techniques, and was then translated to a simplified biomarker to perform on a sample of whole-blood.46 Currently, the simplified whole-blood biomarker is being assessed for clinical utility in the first precision medicine randomized clinical trial (RCT) in IBD, the “Predicting outcomes for Crohn’s disease using a molecular biomarker” (PROFILE) trial.47 There has been some conflicting evidence about whether this blood-based biomarker can also be replicated in additional cohorts including from the paediatric setting, however these findings have highlighted the need for bioinformatic expertise to analyse such datasets and the general need for further validation in larger and more diverse cohorts.48
Ultimately, whichever prognostic tool is used to aid clinical decision-making in the future, it is likely to be of most benefit if used as close to the time of diagnosis as possible—to both guide therapeutic decisions and timing of follow-up. A possible further application of prognostic biomarkers may be to aid selection of patients where it is appropriate to discuss about withdrawal of immunosuppressant, biologic or small molecule therapies.49 In this regard, results from the STORI prospective, observational study of 115 patients with CD, are promising, where initial proteomic data have suggested that a 17-protein panel is able to predict medium and longer-term likelihood of relapse following withdrawal of anti-TNF therapy.50
Prediction of Response
After many decades of limited treatment options for patients with IBD, there is now a hugely promising and large pipeline of potential new treatments.7 Although in other fields such as oncology, companion biomarkers have been critical as part of the early-phase drug development program of novel therapeutics,51 this has only more recently been the case for early drug development programs in IBD.52 In the search for predictors of response, the initial focus in IBD was on models consisting of simple clinical variables available in most health care settings. Although these simple clinical variables have been somewhat informative, they have not proved sufficiently predictive to help accurately guide most treatment decisions.53 Given the time and wealth of experience with antibodies to tumor necrosis factor (anti-TNF), it is perhaps no surprise that the most promising predictive biomarkers have been developed for anti-TNF response and nonresponse. One such predictive application has examined the use of fluorescence targeting to highlight cells with membrane-bound TNF in 25 patients with CD prior to starting anti-TNF treatment.54 Patients with a greater number of fluorescently detected cells had greater response at 12 weeks compared with those with fewer cells detected (92% vs 15%). More recently, this same method has demonstrated similar results with a4b7-expressing cells prior to starting vedolizumab therapy.55 This technology requires further assessment and validation in external cohorts, but the concept of combining endoscopic procedures with real-time imaging to guide clinical treatment is a very enticing and pragmatic prospect for many gastroenterologists.
Perhaps the finding closest to clinical application in this field comes from the Personalising Anti-TNF Therapy in Crohn’s Disease (PANTS) study of 1240 biologic-naïve patients with CD from the UK, which has demonstrated that variants in the HLA-DQA1*05 allele are associated with greater likelihood of immunogenicity and that this immunogenicity can be greatly reduced by use of a concomitant immunomodulator.56 In terms of clinical application, these results would be consistent with a trial of 90 patients with IBD and immune-mediated loss of response (LOR) to a first anti-TNF.57 This RCT concluded that use of an immunomodulator should almost always be recommended when considering switching to a second anti-TNF agent after LOR to a first agent, unless there is a clear contraindication.57 Although HLA-DQA1*05 variants were not assessed in this trial, it seems quite possible that the addition of an immunomodulator reduced risk in much the same way as observed in the PANTS study. Subsequent work is required to assess whether the increased immunogenicity with HLA-DQA1*05 variants is indeed associated with longer-term clinical outcomes and whether modifying treatment based on this biomarker could result in improved outcomes for patients.
A number of further exciting biomarkers in IBD have also focused on gene expression. Indeed, analysis of colonic tissue has demonstrated association of higher triggering receptor expressed on myeloid cells 1 (TREM-1) levels in anti-TNF nonresponders.58 There has been some discordance about whether gene expression of TREM-1 in blood might also be upregulated in anti-TNF nonresponders,59 highlighting the importance of harmonizing definitions of response and nonresponse in the IBD field and the need to harmonize assays for future, larger validation cohorts.
More recent work has aimed to combine different -omic technologies to identify groups that might benefit from different therapeutic options available in IBD.60 Indeed, combining single-cell approaches with gene expression has allowed biological associations to be explored in much greater detail than ever before, revealing a cellular module in inflamed tissue containing IgG plasma cells, inflammatory mononuclear phagocytes, activated T cells, and stromal cells (named GIMATS), associated with anti-TNF nonresponse in 4 independent cohorts.61 This GIMATS module also replicated the finding that oncostatin M (OSM), a stromal activator in IBD, plays a key role in determining nonresponse to anti-TNF in IBD,62 with this OSM signaling process being mostly driven by monocytes in IBD.63 Work is ongoing and needed to ultimately translate the OSM signature into a simple, easy to measure test in clinical practice.64
Looking ahead to the future, perhaps the most promising application for response and nonresponse prediction will come from combining these novel techniques with readily available histopathological information. The greater granularity from such spatial analyses have already been used to identify distinct pathotypes based on localized tissue analysis, and further such application will likely allow more precise use of both current and future therapeutics.65
Prediction of Safety
A major issue that blights clinical practice is the high rate of intolerance and side effects associated with most of the medications in IBD, particularly with immunomodulator treatments. Therefore, a key additional aspect underpinning choice of therapy is the likelihood of potential side effects for each individual patient. Indeed, biomarker testing to predict safety risks have been used in the IBD field for many decades. It is generally recommended to assess for thiopurine methyltransferase (TPMT) genotyping or enzyme activity prior to initiation of thiopurine medications; however, it is important to note that the benefits of such an approach have never been demonstrated in an RCT-setting.
Given that there are now much more advanced testing techniques available, attention has turned to whether these could be applied to predict intolerance or side effects to medications. Although the limited ability to use SNP associations to guide prognostic or predictive testing was highlighted earlier, the one major exception to this is in the field of pharmacogenetics. The potential for application of genetic safety biomarkers in IBD was initially demonstrated by the association of HLA-DQA1 and HLA-DRB1 haplotypes with development of thiopurine-induced pancreatitis.66 Similarly, variants in the HLA region were also shown to associate with the subsequent development of 5-aminosalicylate-induced nephrotoxicity.67 More recently, a nonsynonymous SNP in the gene nudix hydroxylase 15 (NUDT15) has been associated with thiopurine-induced myelosuppression (TIM), initially in a South Korean cohort with an odds ratio of over 35.68 The genetic variant in NUDT15 and its association with TIM has subsequently been replicated in a cohort of European ancestry,69 suggesting that in addition to TPMT testing, NUDT15 might be routinely assessed prior to initiation of a thiopurine medication. The major next step for pharmacogenomics in IBD will be to determine whether there are similar predictive markers available to predict intolerance or side effects to more novel biologic and/or small molecule agents.
The growing optimism for precision medicine can be tempered by the fact that this topic has been discussed for many years now, with little impact on clinical practice to date. Historically, the major barrier to implementation was a lack of prospective validation for promising biomarkers and models. Although this remains important, there is now a growing understanding of the need for validation across diverse populations. This problem is exemplified by historical GWASs, which were typically performed in populations of predominantly European origin.70 However, the importance and power of performing studies in diverse populations has now been well illustrated by a recent meta-GWAS of IBD patients from 3 distinct ethnic origin backgrounds.71 This multiethnic, meta-GWAS found that prediction using PRS was much improved when using data from distinct ancestral populations compared with prediction using data from those with European ancestry alone.
For those precision medicine tools that are shown to be validated and to have clinical utility in diverse populations, perhaps the greatest future challenge lies with interpreting information provided by combinations of biomarkers and selecting from combinations of treatments. Therefore, it seems that the success of clinical adoption will almost certainly depend on development of user-friendly, decision-support systems that are able to combine this myriad of data and aid decision-making by clinicians and patients.
Anecdotally, some clinical teams around the world have already begun implementing some of these novel biomarker tests prior to initiation of therapy. However, there are key considerations for each organization and health care system to consider before adoption of any such biomarkers into routine clinical practice. These include cost, availability of testing, type of testing, interactions affecting test results, the strength of evidence for analytical utility of a biomarker, and most importantly, the clinical utility (ie, whether making treatment decisions based on these biomarkers actually results in improved clinical outcomes for patients).26 It is very likely that different thresholds will need to be applied for biomarkers predictive of safety outcomes, where there is potentially more to gain from avoiding harm to patients than the higher threshold predictive and prognostic biomarkers will have to achieve to be adopted into routine clinical practice.
Early Treatment
Paralleling the growing number of therapeutic options available for IBD, a therapeutic ceiling has emerged whereby even the most effective interventions only result in noticeable improvements for approximately 30% of patients.72 This has led many in the field to conclude that perhaps treatments are being initiated at too late a stage and that earlier initiation of appropriate treatment may allow us to break through this therapeutic ceiling.72
This is well-illustrated by a growing body of evidence across clinical trials, including post hoc, longer-term analyses and real-world observational studies demonstrating improved outcomes with earlier initiation of treatments. Although it must be noted that the majority of evidence has been generated in CD, with comparatively less data for UC at this stage.73
The “step-up, top-down” trial of 133 patients with recently diagnosed CD demonstrated that early combined immunosuppression with infliximab and azathioprine resulted in improved clinical remission at 52 weeks (62% vs 42%).74 However, there was a recognition that the indiscriminate use of top-down treatment in all patients from diagnosis would lead to the risk of overimmunosuppression in some patients and would not at the time have been affordable in almost any health care setting in the world. Accordingly, the Randomised Evaluation of an Algorithm for Crohn’s Treatment (REACT-1) cluster RCT of 41 community centers caring for 1982 patients with CD investigated whether a more accelerated step-up approach using early combined immunosuppression could be used instead.75 Although REACT-1 did not demonstrate a significant improvement in the primary outcome of clinical remission compared with conventional treatment, an advantage of the accelerated step-up approach was demonstrated across many major secondary outcome measures at 24 months, including risks for hospital admission, need for surgery, or any serious CD-related complication.75 Similarly post hoc analysis from the CALM trial comparing early combined immunosuppression and tight control of disease showed improved endoscopic outcomes at 1 year following trial completion.76
The findings across these pivotal trials highlight the critical importance of primary endpoint selection in RCTs and that there is still a lack of prospective clinical trial data with long-term primary outcomes in IBD. In the longer-term, the solution to this will be to convince funders, regulators, clinicians, and patients to conduct and complete longer-term RCTs in IBD.
Despite findings from post hoc analyses of RCTs, there have been conflicting data around early treatment benefits from real-world observational cohorts. Although some observational cohorts have also reported improved outcomes with earlier initiation of treatment, including anti-TNF treatment,77 other studies have not demonstrated any significant differences in major clinical outcomes despite greater use of more advanced therapies.78 One reason put forward for this discrepancy in findings is that many of these treatments may not have been used “at the right time” and perhaps not early enough in the disease course to make a difference to individual patients. Indeed in REACT-1, the typical disease duration for enrolled patients was 3 years from diagnosis. Even in the CALM trial, where at best only 45% of patients could achieve endoscopic remission by the end of week 48, the median time from diagnosis to enrolment was still almost 12 months.
One of the difficulties in IBD has been a lack of agreement over what constitutes early IBD. There has been a growing recognition that initial suggestions of a 2-year window from diagnosis79 are not stringent enough and that perhaps we should be more in line with analogous fields such as rheumatology, where the window for early rheumatoid arthritis has been defined as early as 3 months from diagnosis.80
An area of urgent research priority in the field is to better understand the positioning of newer agents in the therapeutic algorithms of IBD. Most novel therapies have been investigated predominantly in the setting of intolerance or loss of response to anti-TNF therapy, with decreased effectiveness of treatments being observed when used as second-, third-, and fourth-line options.81 As such, important unanswered questions remain about how best to sequence therapies in IBD and whether earlier effective treatment may also mean greater use of novel combinations, as demonstrated in fields such as oncology.82 Although there is certainly some early signals of effect from combination therapies,83 the true efficacy and especially safety of this approach needs to be explored in more detail, ideally through well-conducted RCTs.
Even though the focus has been on early medical treatments, it is important to acknowledge the significance of early surgery where this is appropriate; and for certain cases, this may well offer the best clinical outcomes for a patient in both the short and longer term. With respect to early surgery in IBD, the seminal LIR!C trial demonstrated no difference in clinical outcomes with regard to anti-TNF biologic medication compared with ileocecal resection for 143 patients with ileocecal CD who were refractory to immunomodulator treatment.84 Early surgery in such cases was shown to be a cost-effective option85 and had comparable outcomes even in longer-term follow-up for over 5 years.86 It must be noted that there are many other factors that have been associated with better surgical outcomes for patients with IBD including availability of laparoscopic access87 and care in a high-volume surgical center.88 However going forward, early discussions about availability and appropriateness of surgery is likely to form a key part of a more personalized approach to patient care.
Early Tight Monitoring
Tight Control
It has long been understood that CD is a progressive disease with irreversible bowel damage.89 More recently, the progressive nature of UC with structural and functional damage has also been recognized,90 leading many to conclude that UC has a similar disease burden and requires similar treatment goals.91 Over time, these treatment goals have evolved in IBD; and most recently, the STRIDE group updated recommendations for treating-to-target in IBD, establishing short, intermediate, and long-term targets and incorporating patient preferences to guide therapeutic decision-making.92
The STRIDE-II group identified the ultimate long-term goal for treatment as deep remission of disease, with a combination of clinical, endoscopic, histological, and transmural healing. This concept has also been termed disease clearance93 but may not be achievable in many patients. Indeed, further research is needed to determine if the potential gains obtained with disease clearance are worth the costs and potential adverse events in pursuit of this target. Notwithstanding these factors, there is much evidence in favor of early disease control guided by active monitoring. The CALM trial of 244 patients with CD who were naïve to immunomodulator and biologics demonstrated that tight control with timely treatment escalation based on C-reactive protein (CRP) and calprotectin targets resulted in improved endoscopic healing and improved clinical outcomes compared with treatment strategies driven by symptoms alone (46% vs 30%).94 Similarly in the Postoperative Crohn’s Endoscopic Recurrence (POCER) trial, which assessed the optimal strategy to prevent postoperative CD recurrence at 18 months, active control with early colonoscopy and treatment for endoscopic recurrence was superior to conventional treatment reactive to symptoms alone (49% vs 67%).95 Analysis of stool samples obtained from the POCER trial demonstrated that fecal calprotectin levels above 100 μg/g were associated with early endoscopic recurrence, suggesting that use of noninvasive fecal calprotectin levels could be used to help further guide timing for endoscopy and better direct postoperative treatment.96
One note of caution should perhaps be considered with regard to tight control, as demonstrated by the STARDUST trial (Study of Treat to Target Versus Routine Care Maintenance Strategies in Crohn’s Disease Patients Treated With Ustekinumab; NCT03107793) of 500 patients. In STARDUST, a tight monitoring strategy resulted in clinical improvement, but the intention-to-treat analysis demonstrated no statistically significant improvements in more objective outcome measures such as endoscopic healing.
It is certainly also worth highlighting the importance of the microbiome in IBD,97 and recent work has suggested the potential use of microbial, metabolomic, and proteomic profiles to predict relapse in CD and UC.98 Similarly, perhaps the most promising work to date in this field has suggested a potential for microbiota to specifically predict postoperative relapse in CD.99 Nevertheless, one challenge remains: the individual microbiome for every patient is dynamic and constantly changing in response to environmental stimuli, making it difficult to standardize testing in order to personalize treatment approaches.
Even though the evidence for tight monitoring in IBD exists mainly for CD, there is certainly rationale that it may also be beneficial in UC,100 and we look forward to the results from the ongoing, international VERDICT trial (NCT04259138), which is the first tight control trial in UC trying to determine the optimal treatment target (clinical vs clinical and endoscopic vs clinical, endoscopic and histologic remission).
Both the SPIRIT and STRIDE-II consensus recommendations highlighted further promising options, such as the potential for noninvasive imaging, to monitor disease and support tight control.92, 101 The initial focus of noninvasive imaging in IBD was mostly on magnetic resonance enterography (MRE) and predominantly focused on CD. In a cohort of 48 CD patients, MRE was shown to have a high level of accuracy in assessing response to treatment and endoscopic healing when using ileocolonoscopy as the reference standard.102 More recently, there has been enthusiasm about the potential application of ultrasound in the assessment of IBD activity and response to treatment. Ultrasound is cheaper, is potentially much more readily available, and can be repeatedly performed or even used as a point-of-care test. The METRIC trial including 284 patients with newly diagnosed or relapsed CD compared the diagnostic accuracy of MRE and small bowel ultrasound for the extent and activity of disease; the study demonstrated good accuracy of both these methods in expert hands (MRI 97% and ultrasound 92% sensitivity for presence of inflammation).103 Bowel ultrasonography has since been demonstrated to be an accurate monitoring modality in the context of different therapeutic agents in CD104 and was also found to be helpful in monitoring UC disease course and treatment response.105
Therapeutic Drug Monitoring
In addition to patient stratification and initial selection of treatment, precision medicine can also be used to monitor and optimize treatment through therapeutic drug monitoring (TDM). The measurement of metabolites from thiopurine has long been available in clinical practice, with optimal levels including thresholds for likely toxicity having been defined.106 In recent years, there have also been numerous observational studies suggesting additional value of TDM for more novel agents such as biologic medications,107 with the most evidence to date investigating anti-TNF therapies.108 Although there is growing evidence for newer agents such as ustekinumab and vedolizumab, given their relative infancy of use in IBD, the utility of TDM for these medications is still yet to be determined.109
Most studies supporting use of TDM have investigated a reactive approach, but the PANTS study highlighted the potential importance of proactive TDM—where the only clinical predictor that was reliably able to predict anti-TNF nonresponse at 1 year was low drug concentrations observed following induction.110 In addition, data mostly from observational studies have further supported a potential role for proactive TDM.111 However, it is important to note that evidence from RCTs and the use of proactive TDM have not been so promising. The TAXIT trial, an RCT evaluating 263 patients with both UC and CD starting infliximab treatment, revealed no difference in clinical remission at 1 year for concentration-based dosing compared with standard clinical dosing.112 Nevertheless, subsequent post hoc analysis of TAXIT suggested that high antibody titres that persist over time may be more clinically relevant and that patients in this subgroup should perhaps be focused on and included for future studies.113
Similarly to TAXIT, the prospective TAILORIX trial including 122 anti-TNF-naïve CD patients starting infliximab therapy was not able to demonstrate the superiority of concentration-based infliximab dosing compared with dosing based on symptoms at 1 year.114 In contrast to the RCT evidence for adult patients, the PAILOT trial of 78 pediatric patients with CD who were treated with adalimumab was the first to demonstrate in an RCT-setting that proactive monitoring of trough concentrations with adjustment of adalimumab-dosing resulted in higher rates of steroid-free clinical remission in comparison with reactive monitoring (82% vs 48%).115
The inability to demonstrate benefit for proactive TDM across RCTs in adults with IBD may be a consequence of limitations from the described trials. TAILORIX was likely underpowered to demonstrate differences between the approaches used, and dose intensification could only commence after week 14 of the trial schedule. An earlier dose modification might have resulted in differences being noted across key clinical outcomes. Accordingly, in a recent prospective study of 108 IBD patients starting anti-TNF medication, the detection of antibodies to anti-TNF with a drug-tolerant assay, as early as week 2, was predictive of later treatment failure within 24 months.116 However, such early use of tests for anti-TNF level and antibody determination is currently limited by the duration of time to obtain results in most countries. The availability of validated assays that allow rapid point-of-care measurement for both levels and antibodies would allow earlier use of TDM following treatment initiation; this would provide more robust opportunities to evaluate the impact of TDM on clinical outcomes—namely the prevention of primary nonresponse.117, 118
The existing evidence is still insufficient to support routine proactive TDM in adult patients with IBD. Nevertheless, it seems that for now at least, reactive TDM will continue to be a useful tool available for clinicians, particularly in the setting of LOR. It should also be noted that despite most published studies focusing on trough levels of anti-TNF, this parameter fails to provide the whole pharmacokinetic picture. Future studies will need to explore other parameters such as area under the concentration-time curve, clearance, and average concentrations to obtain a greater understanding about TDM. A further concept that will likely become more familiar over time are dashboards (ie, software systems that can integrate population-based pharmacokinetic models with information collected from TDM and patient factors). These dashboard systems are likely to be invaluable tools to guide clinical practice in the future, but prospective investigation and particularly RCT evaluation will be required to demonstrate clinical utility of such systems.119
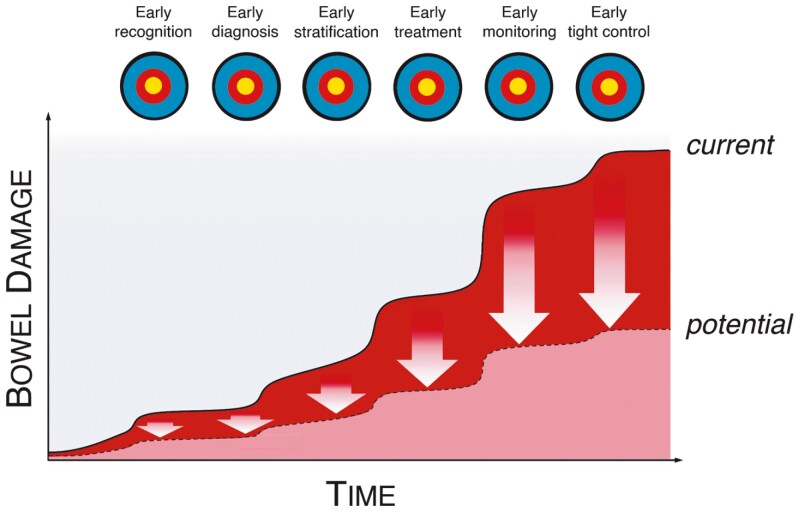
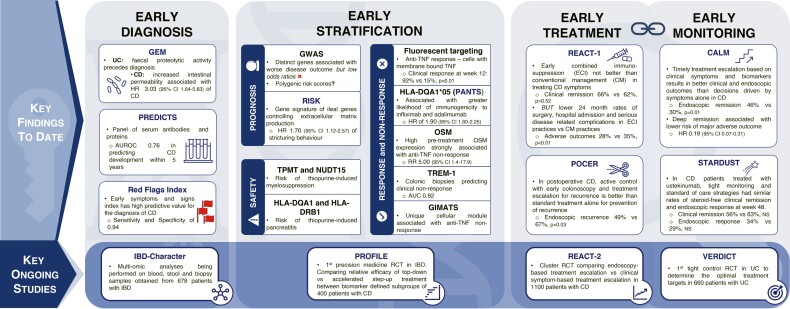
We believe that a combination of all the early actions described in this article (early recognition, early diagnosis, early stratification, early treatment, early monitoring, and early tight control) combined with the novel precision medicine tools highlighted will lead to greatly reduced bowel damage, fewer complications, and improved outcomes for patients with IBD (Figure 2). We also present summary of key findings from studies to date, in addition to consideration of the most promising studies to help inform future integration of precision medicine tools with early care and early action in IBD (Figure 3).
Figure 2.
Diagram to show how early action can improve clinical outcomes for patients living with IBD. Highlighting the importance of early recognition, early diagnosis, early stratification, early treatment, early and tight monitoring, and early intervention based on monitoring results to ultimately reduce longer-term bowel damage and ensure better control of IBD for patients.
Figure 3.
Diagram to summarize data from the most promising current and future studies exploring early diagnosis, early stratification, early treatment, and monitoring. Highlighting findings from the key studies to date on early diagnosis, stratification, treatment, and monitoring. Subsequently highlighting the most promising upcoming studies, which will provide key data to support further progress in these topics.
Conclusions and Future Directions
There has been much excitement about novel precision medicine tools and their potential to improve outcomes for many patients. Indeed, there are various precision medicine tools already validated or undergoing validation which offer real hope for clinical application in the near future. However, it is important to restate that the goal of precision medicine is to deliver improved outcomes for each individual patient.
Even with the availability of all these precision tools, clinicians should bear in mind that these tools must be combined with a focus on early action. This will involve early recognition to enable timely diagnosis, early stratification to guide early treatment selection, and crucially early monitoring to ensure tight control of disease activity.
Earlier recognition and referral to specialists from primary care are likely to be aided by simple measures such as use of red flag systems and increasing education for both the medical community and the public alike. Earlier assessment and diagnosis will likely be aided by simpler and more convenient investigations such as home calprotectin testing120 or point-of-care calprotectin testing.121 Additionally, wider availability and affordability of biosimilar drugs122 should result in earlier initiation of more effective therapies. However, earlier treatment will also need to account for convenience of therapies for patients, highlighting the need for more effective subcutaneous and oral treatment options that patients are able to take in their own home. Following diagnosis and initiation of treatment, early and tight monitoring is essential. In the future, this monitoring is likely to be through a combination of multiple parameters such as clinical data, biomarkers, imaging, TDM modeling, and simulation.
The optimal way to integrate the growing numbers of biomarkers, prediction tools, and therapeutics in IBD is an enormous challenge. Clinical decision support tools harnessing big data and offering simple, easy to understand options to patients and clinicians are therefore necessary and will be crucial. Combining early action in IBD with these novel tools likely offers the greatest opportunity to deliver precision medicine in IBD. Focusing efforts in this area will hopefully ensure that we can soon achieve the goal of getting the right treatment to the right patient at the right time of their individualized disease course.
Acknowledgments
None.
Contributor Information
Nurulamin M Noor, Department of Gastroenterology, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Trust, Cambridge, United Kingdom; Medical Research Council Clinical Trials Unit, University College London, London, United Kingdom.
Paula Sousa, Department of Gastroenterology, Viseu Unit, Tondela-Viseu Hospital Centre, 3504–509 Viseu, Portugal.
Stéphane Paul, Faculty of Medicine of Saint-Etienne, Immunology Unit University Hospital of Saint-Etienne, CIC INSERM 1408, Saint-Etienne, France.
Xavier Roblin, Department of Gastroenterology, University Hospital of Sain- Etienne, Saint-Etienne, France.
Author Contributions
N.M.N., P.S., S.P., and X.R. conceived the idea for this manuscript and were responsible for the planning, content, and structure of the article. N.M.N. and P.S. wrote the initial manuscript draft with input from S.P. and X.R. All authors provided critical revisions and approved the final version of the manuscript.
Funding
There is no specific funding associated with the development of this manuscript. N.M.N. is supported by a Medical Research Council PhD Studentship (MC_UU_171339).
Conflicts of Interest
The authors declare no competing financial interests relevant to this work.
References
- 1. Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burisch J, Pedersen N, Čuković-Čavka S, et al. ; EpiCom-group . East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588–597. [DOI] [PubMed] [Google Scholar]
- 4. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 5. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. [DOI] [PubMed] [Google Scholar]
- 6. Agrawal M, Sabino J, Frias-Gomes C, et al. Early life exposures and the risk of inflammatory bowel disease: systematic review and meta-analyses. Eclinicalmedicine. 2021;36:100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paramsothy S, Rosenstein AK, Mehandru S, Colombel JF. The current state of the art for biological therapies and new small molecules in inflammatory bowel disease. Mucosal Immunol. 2018;11:1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fiocchi C, Dragoni G, Iliopoulos D, et al. Results of the seventh scientific workshop of ECCO: precision medicine in IBD—what, why, and how. J Crohn’s Colitis. 2021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9. Nahon S, Lahmek P, Lesgourgues B, et al. Diagnostic delay in a French cohort of Crohn’s disease patients. J Crohns Colitis. 2014;8:964–969. [DOI] [PubMed] [Google Scholar]
- 10. Irving P, Burisch J, Driscoll R, et al. IBD2020 global forum: results of an international patient survey on quality of care. Intest Res. 2018;16:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker GJ, Lin S, Chanchlani N, et al. Quality improvement project identifies factors associated with delay in IBD diagnosis. Aliment Pharmacol Ther. 2020;52:471–480. [DOI] [PubMed] [Google Scholar]
- 12. Barratt SM, Leeds JS, Robinson K, et al. Prodromal irritable bowel syndrome may be responsible for delays in diagnosis in patients presenting with unrecognized Crohn’s disease and celiac disease, but not ulcerative colitis. Dig Dis Sci. 2011;56:3270–3275. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen VQ, Jiang D, Hoffman SN, et al. Impact of diagnostic delay and associated factors on clinical outcomes in a U.S. inflammatory bowel disease cohort. Inflamm Bowel Dis. 2017;23:1825–1831. [DOI] [PubMed] [Google Scholar]
- 14. Blackwell J, Saxena S, Jayasooriya N, et al. Prevalence and duration of gastrointestinal symptoms before diagnosis of inflammatory bowel disease and predictors of timely specialist review: a population-based study. J Crohn’s Colitis. 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez-Lago I, Agirre U, Intxaurza N, et al. Increased use of healthcare resources during the preclinical period of inflammatory bowel disease. Dig Liver Dis. 2021;53:927–930. [DOI] [PubMed] [Google Scholar]
- 16. Irwin JR, Ferguson E, Simms LA, et al. Detectable laboratory abnormality is present up to 12 months prior to diagnosis in patients with Crohn’s disease. Dig Dis Sci. 2019;64:503–517. [DOI] [PubMed] [Google Scholar]
- 17. Danese S, Fiorino G, Mary JY, et al. Development of red flags index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: an IOIBD initiative. J Crohns Colitis. 2015;9:601–606. [DOI] [PubMed] [Google Scholar]
- 18. Fiorino G, Bonovas S, Gilardi D, et al. Validation of the red flags index for early diagnosis of Crohn’s disease: a prospective observational IG-IBD study among general practitioners. J Crohn’s Colitis. 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 19. Torres J, Burisch J, Riddle M, et al. Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut. 2016;65:1061–1069. [DOI] [PubMed] [Google Scholar]
- 20. Torres J, Petralia F, Sato T, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology. 2020;159:96–104. [DOI] [PubMed] [Google Scholar]
- 21. Galipeau HJ, Caminero A, Turpin W, et al. ; CCC Genetics, Environmental, Microbial Project Research Consortium . Novel fecal biomarkers that precede clinical diagnosis of ulcerative colitis. Gastroenterology. 2021;160:1532–1545. [DOI] [PubMed] [Google Scholar]
- 22. Turpin W, Lee SH, Raygoza Garay JA, et al. ; Crohn’s and Colitis Canada Genetic Environmental Microbial Project Research Consortium; CCC GEM Project recruitment site directors include Maria Abreu . Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology. 2020;159:2092–2100.e5. [DOI] [PubMed] [Google Scholar]
- 23. Ventham NT, Kennedy NA, Adams AT, et al. ; IBD BIOM consortium; IBD CHARACTER consortium . Integrative epigenome-wide analysis demonstrates that DNA methylation may mediate genetic risk in inflammatory bowel disease. Nat Commun. 2016;7:13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torres J, Halfvarson J, Rodríguez-Lago I, et al. Results of the Seventh Scientific Workshop of ECCO: Precision medicine in IBD- prediction and prevention of inflammatory bowel disease. J Crohn’s Colitis. 2021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 25. Bolte LA, Vich Vila A, Imhann F, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verstockt B, Noor NM, Marigorta UM, et al. Results of the Seventh Scientific Workshop of ECCO: Precision medicine in IBD—disease outcome and response to therapy. J Crohn’s Colitis. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flamant M, Roblin X. Inflammatory bowel disease: towards a personalized medicine. Therap Adv Gastroenterol. 2018;11:1756283X17745029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noor NM, Verstockt B, Parkes M, Lee JC. Personalised medicine in Crohn’s disease. Lancet Gastroenterol Hepatol. 2020;5:80–92. [DOI] [PubMed] [Google Scholar]
- 29. Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–656. [DOI] [PubMed] [Google Scholar]
- 30. Torres J, Caprioli F, Katsanos KH, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis. 2016;10:1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamb CA, Kennedy NA, Raine T, et al. ; IBD guidelines eDelphi consensus group . British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solberg IC, Vatn MH, Høie O, et al. ; IBSEN Study Group . Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. [DOI] [PubMed] [Google Scholar]
- 33. Wintjens D, Bergey F, Saccenti E, et al. Disease activity patterns of Crohn’s disease in the first 10 years after diagnosis in the population-based IBD South Limburg cohort. J Crohn’s Colitis. 2021;15:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paul S, Boschetti G, Rinaudo-Gaujous M, et al. Association of anti-glycan antibodies and inflammatory bowel disease course. J Crohns Colitis. 2015;9:445–451. [DOI] [PubMed] [Google Scholar]
- 35. Whittle R, Royle KL, Jordan KP, et al. Prognosis research ideally should measure time-varying predictors at their intended moment of use. Diagn Progn Res. 2017;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riley RD, Hayden JA, Steyerberg EW, et al. ; PROGRESS Group . Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. Plos Med. 2013;10:e1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choung RS, Princen F, Stockfisch TP, et al. ; PREDICTS Study Team . Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment Pharmacol Ther. 2016;43:1300–1310. [DOI] [PubMed] [Google Scholar]
- 38. Lee JC, Biasci D, Roberts R, et al. ; UK IBD Genetics Consortium . Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn’s disease. Nat Genet. 2017;49:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haritunians T, Taylor KD, Targan SR, et al. Genetic predictors of medically refractory ulcerative colitis. Inflamm Bowel Dis. 2010;16:1830–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen GB, Lee SH, Montgomery GW, et al. ; International IBD Genetics Consortium . Performance of risk prediction for inflammatory bowel disease based on genotyping platform and genomic risk score method. BMC Med Genet. 2017;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ananthakrishnan AN. IBD risk prediction using multi-ethnic polygenic risk scores. Nat Rev Gastroenterol Hepatol. 2021;18:217–218. [DOI] [PubMed] [Google Scholar]
- 42. Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet. 2017;389:1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haberman Y, Minar P, Karns R, et al. Mucosal inflammatory and wound healing gene programmes reveal targets for stricturing behaviour in paediatric Crohn’s disease. J Crohn’s Colitis. 2020;15:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ungaro RC, Hu L, Ji J, et al. Machine learning identifies novel blood protein predictors of penetrating and stricturing complications in newly diagnosed paediatric Crohn’s disease. Aliment Pharmacol Ther. 2021;53:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest. 2011;121:4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Biasci D, Lee JC, Noor NM, et al. A blood-based prognostic biomarker in IBD. Gut. 2019;68:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parkes M, Noor NM, Dowling F, et al. PRedicting outcomes For Crohn’s dIsease using a moLecular biomarkEr (PROFILE): protocol for a multicentre, randomised, biomarker-stratified trial. BMJ Open. 2018;8:e026767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee JC, Lyons PA, McKinney EF. Analytical mistakes confound attempted validation: a response to “Transcription and DNA Methylation Patterns of Blood-Derived CD8+ T Cells Are Associated With Age and Inflammatory Bowel Disease But Do Not Predict Prognosis”. Gastroenterology. 2021;160:2210–2211. [DOI] [PubMed] [Google Scholar]
- 49. Boyapati RK, Torres J, Palmela C, et al. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn’s disease. Cochrane Database Syst Rev. 2018;5:CD012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pierre N, Baiwir D, Huynh-Thu VA, et al. Discovery of biomarker candidates associated with the risk of short-term and mid/long-term relapse after infliximab withdrawal in Crohn’s patients: a proteomics-based study. Gut. 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 51. Nicolaides NC, O’Shannessy DJ, Albone E, Grasso L. Co-development of diagnostic vectors to support targeted therapies and theranostics: essential tools in personalized cancer therapy. Front Oncol. 2014;4:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sandborn WJ, Vermeire S, Tyrrell H, et al. Etrolizumab for the treatment of ulcerative colitis and Crohn’s disease: an overview of the phase 3 clinical program. Adv. Ther. 2020;37:3417–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology. 2018;155:687–695.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Atreya R, Neumann H, Neufert C, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014;20:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rath T, Bojarski C, Neurath MF, et al. Molecular imaging of mucosal α4β7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn’s disease. Gastrointest. Endosc. 2017;86:406–408. [DOI] [PubMed] [Google Scholar]
- 56. Sazonovs A, Kennedy NA, Moutsianas L, et al. ; PANTS Consortium . HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology. 2020;158:189–199. [DOI] [PubMed] [Google Scholar]
- 57. Roblin X, Williet N, Boschetti G, et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut. 2020;69:1206–1212. [DOI] [PubMed] [Google Scholar]
- 58. Gaujoux R, Starosvetsky E, Maimon N, et al. ; Israeli IBD research Network (IIRN) . Cell-centred meta-analysis reveals baseline predictors of anti-TNFα nonresponse in biopsy and blood of patients with IBD. Gut. 2019;68:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Verstockt B, Verstockt S, Dehairs J, et al. Low TREM1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. EBioMed. 2019;40:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aschenbrenner D, Quaranta M, Banerjee S, et al. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut. 2021;70:1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martin JC, Chang C, Boschetti G, et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178:1493–1508.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. West NR, Hegazy AN, Owens BMJ, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor–neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017;23:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–730.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Verstockt S, Verstockt B, Machiels K, et al. Oncostatin M is a biomarker of diagnosis, worse disease prognosis, and therapeutic nonresponse in inflammatory bowel disease. Inflamm. Bowel Dis. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Friedrich M, Pohin M, Jackson MA, et al. IL-1-driven stromal-neutrophil interaction in deep ulcers defines a pathotype of therapy nonresponsive inflammatory bowel disease. bioRxiv. 2021. Preprint. [Google Scholar]
- 66. Heap GA, Weedon MN, Bewshea CM, et al. ; International Serious Adverse Events Consortium; IBD Pharmacogenetics Study Group . HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014;46:1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heap GA, So K, Weedon M, et al. Clinical features and HLA association of 5-aminosalicylate (5-ASA)-induced nephrotoxicity in inflammatory bowel disease. J Crohns Colitis. 2016;10:149–158. [DOI] [PubMed] [Google Scholar]
- 68. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Walker GJ, Harrison JW, Heap GA, et al. ; IBD Pharmacogenetics Study Group . Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. 2019;321:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gurdasani D, Barroso I, Zeggini E, Sandhu MS. Genomics of disease risk in globally diverse populations. Nat Rev Genet. 2019;20:520–535. [DOI] [PubMed] [Google Scholar]
- 71. Gettler K, Levantovsky R, Moscati A, et al. ; UK IBD Genetics Consortium, National Institute of Diabetes, Digestive and Kidney Diseases Inflammatory Bowel Disease Genetics Consortium . Common and rare variant prediction and penetrance of IBD in a large, multi-ethnic, health system-based biobank cohort. Gastroenterology. 2021;160:1546–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Alsoud D, Verstockt B, Fiocchi C, Vermeire S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol Hepatol. 2021;6:589–595. [DOI] [PubMed] [Google Scholar]
- 73. Danese S, Fiorino G, Peyrin-Biroulet L. Early intervention in Crohn’s disease: towards disease modification trials. Gut. 2017;66:2179–2187. [DOI] [PubMed] [Google Scholar]
- 74. D’Haens G, Baert F, van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club . Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. [DOI] [PubMed] [Google Scholar]
- 75. Khanna R, Bressler B, Levesque BG, et al. ; REACT Study Investigators . Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386:1825–1834. [DOI] [PubMed] [Google Scholar]
- 76. Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology. 2020;159:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Frei R, Fournier N, Zeitz J, et al. Early initiation of anti-TNF is associated with favourable long-term outcome in Crohn’s disease: 10-year-follow-up data from the Swiss IBD cohort study. J Crohns Colitis. 2019;13:1292–1301. [DOI] [PubMed] [Google Scholar]
- 78. Burisch J, Kiudelis G, Kupcinskas L, et al. ; Epi-IBD group . Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019;68:423–433. [DOI] [PubMed] [Google Scholar]
- 79. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut. 2010;59:141–147. [DOI] [PubMed] [Google Scholar]
- 80. Nell VP, Machold KP, Eberl G, et al. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford). 2004;43:906–914. [DOI] [PubMed] [Google Scholar]
- 81. Bressler B, Yarur A, Silverberg MS, et al. Vedolizumab and anti-TNFα real-world outcomes in biologic-naïve inflammatory bowel disease patients: results from the EVOLVE study. J Crohn’s Colitis. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Doroshow JH, Simon RM. On the Design of Combination Cancer Therapy. Cell. 2017;171:1476–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahmed W, Galati J, Kumar A, et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 84. Ponsioen CY, de Groof EJ, Eshuis EJ, et al. ; LIR!C study group . Laparoscopic ileocecal resection versus infliximab for terminal ileitis in Crohn’s disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol. 2017;2:785–792. [DOI] [PubMed] [Google Scholar]
- 85. de Groof EJ, Stevens TW, Eshuis EJ, et al. ; LIR!C study group. Cost-effectiveness of laparoscopic ileocaecal resection versus infliximab treatment of terminal ileitis in Crohn’s disease: the LIR!C Trial. Gut. 2019;68:1774–1780. [DOI] [PubMed] [Google Scholar]
- 86. Stevens TW, Haasnoot ML, D’Haens GR, et al. ; LIR!C study group. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: retrospective long-term follow-up of the LIR!C trial. Lancet Gastroenterol Hepatol. 2020;5:900–907. [DOI] [PubMed] [Google Scholar]
- 87. Dasari BV, McKay D, Gardiner K. Laparoscopic versus open surgery for small bowel Crohn’s disease. Cochrane Database Syst. Rev. 2011;1:CD006956. [DOI] [PubMed] [Google Scholar]
- 88. Egberg MD, Galanko JA, Kappelman MD. Patients who undergo colectomy for pediatric ulcerative colitis at low-volume hospitals have more complications. Clin Gastroenterol Hepatol. 2019;17:2713–2721.e4. [DOI] [PubMed] [Google Scholar]
- 89. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. [DOI] [PubMed] [Google Scholar]
- 90. Torres J, Billioud V, Sachar DB, et al. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356–1363. [DOI] [PubMed] [Google Scholar]
- 91. Berre CL, Ananthakrishnan AN, Danese S, et al. Ulcerative colitis and Crohn’s disease have similar burden and goals for treatment. Clin. Gastroenterol. Hepatol. 2020;18:14–23. [DOI] [PubMed] [Google Scholar]
- 92. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD . STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. [DOI] [PubMed] [Google Scholar]
- 93. Danese S, Roda G, Peyrin-Biroulet L. Evolving therapeutic goals in ulcerative colitis: towards disease clearance. Nat Rev Gastroenterol Hepatol. 2020;17:1–2. [DOI] [PubMed] [Google Scholar]
- 94. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 95. Cruz PD, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406–1417. [DOI] [PubMed] [Google Scholar]
- 96. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938–947.e1. [DOI] [PubMed] [Google Scholar]
- 97. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. ; IBDMDB Investigators . Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borren NZ, Plichta D, Joshi AD, et al. Multi-omics profiling in patients with quiescent inflammatory bowel disease identifies biomarkers predicting relapse. Inflamm. Bowel Dis. 2020;26:1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sokol H, Brot L, Stefanescu C, et al. ; REMIND Study Group Investigators . Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut. 2020;69:462–472. [DOI] [PubMed] [Google Scholar]
- 100. Ungaro R, Colombel J-F, Lissoos T, et al. A treat-to-target update in ulcerative colitis. Am. J. Gastroenterol. 2019;114:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Berre CL, Peyrin-Biroulet L, Sandborn WJ, et al. Selecting end points for disease-modification trials in inflammatory bowel disease: the SPIRIT consensus from the IOIBD. Gastroenterology. 2021;160:1452–1460. [DOI] [PubMed] [Google Scholar]
- 102. Ordás I, Rimola J, Rodríguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374–82.e1. [DOI] [PubMed] [Google Scholar]
- 103. Taylor SA, Mallett S, Bhatnagar G, et al. ; METRIC study investigators . Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Calabrese E, Rispo A, Zorzi F, et al. Ultrasonography tight control and monitoring in Crohn’s disease during different biological therapies: a multicenter study. Clin. Gastroenterol. Hepatol. 2021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 105. Maaser C, Petersen F, Helwig U, et al. ; German IBD Study Group and the TRUST&UC study group; German IBD Study Group and TRUST&UC study group . Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut. 2020;69:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sousa P, Estevinho MM, Dias CC, et al. Thiopurines’ metabolites and drug toxicity: a meta-analysis. J. Clin. Med. 2020;9:2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1655–1668.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dreesen E, Baert F, Laharie D, et al. Monitoring a combination of calprotectin and infliximab identifies patients with mucosal healing of Crohn’s disease. Clin Gastroenterol Hepatol. 2020;18:637–646.e11. [DOI] [PubMed] [Google Scholar]
- 109. Restellini S, Afif W. Update on TDM (therapeutic drug monitoring) with ustekinumab, vedolizumab and tofacitinib in inflammatory bowel disease. J. Clin. Med. 2021;10:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kennedy NA, Heap GA, Green HD, et al. ; UK Inflammatory Bowel Disease Pharmacogenetics Study Group . Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–353. [DOI] [PubMed] [Google Scholar]
- 111. Roblin X, Riviere P, Flamant M, et al. Proactive therapeutic drug monitoring of TNF antagonists in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:1904–1909. [DOI] [PubMed] [Google Scholar]
- 112. Casteele NV, Ferrante M, Assche GV, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–1329. [DOI] [PubMed] [Google Scholar]
- 113. Stappen TV, Casteele NV, Assche GV, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67:818–826. [DOI] [PubMed] [Google Scholar]
- 114. D’Haens G, Vermeire S, Lambrecht G, et al. ; GETAID . Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn’s disease. Gastroenterology. 2018;154:1343–1351.e1. [DOI] [PubMed] [Google Scholar]
- 115. Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn’s disease compared with reactive monitoring. Gastroenterology. 2019;157:985–996.e2. [DOI] [PubMed] [Google Scholar]
- 116. Tournier Q, Paul S, Williet N, et al. Early detection of anti-drug antibodies during initiation of anti-tumour necrosis factor therapy predicts treatment discontinuation in inflammatory bowel disease. Aliment Pharmacol Ther. 2021;53:1190–1200. [DOI] [PubMed] [Google Scholar]
- 117. Cherry M, Dutzer D, Nasser Y, et al. Point-of-care assays could be useful for therapeutic drug monitoring of IBD patients in a proactive strategy with adalimumab. J. Clin. Med. 2020;9:2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sparrow MP, Papamichael K, Ward MG, et al. Therapeutic drug monitoring of biologics during induction to prevent primary nonresponse. J Crohns Colitis. 2020;14:542–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Strik AS, Löwenberg M, Mould DR, et al. Efficacy of dashboard driven dosing of infliximab in inflammatory bowel disease patients; a randomized controlled trial. Scand J Gastroenterol. 2021;56:145–154. [DOI] [PubMed] [Google Scholar]
- 120. Bello C, Roseth A, Guardiola J, et al. Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig Liver Dis. 2017;49:991–996. [DOI] [PubMed] [Google Scholar]
- 121. Derwa Y, Williams CJM, Sood R, et al. Factors affecting clinical decision-making in inflammatory bowel disease and the role of point-of-care calprotectin. Therap Adv Gastroenterol. 2018;11:1756283X17744739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ribaldone DG, Saracco GM, Astegiano M, Pellicano R. Efficacy of infliximab biosimilars in patients with Crohn’s disease. Lancet. 2017;390:2435–2436. [DOI] [PubMed] [Google Scholar]