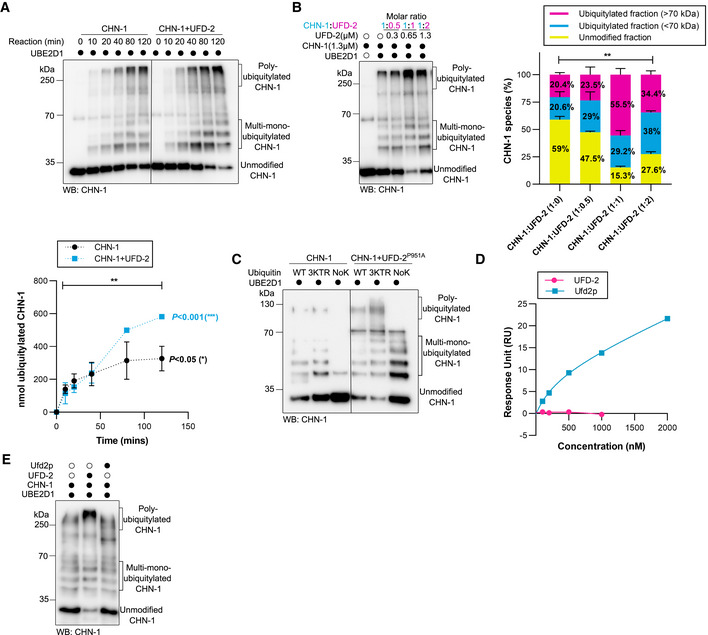
Figure 1. UFD‐2 activates CHN‐1.
-
ATime‐dependent (0, 10, 20, 40, 80, and 120 min) CHN‐1 auto‐Ub was performed as indicated using UbWT and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Below, a graph representing the nmol of ubiquitylated CHN‐1 vs. time for CHN‐1 alone (black) or CHN‐1 + UFD‐2 (cyan). Plotted data are the mean of three technical replicates. Error bars represent the standard error of measurement (SEM); statistical significance was determined using Pearson's correlation coefficients which define the statistical relation between two continuous variables [CHN‐1 vs. time, CHN‐1 + UFD‐2 vs. time, and CHN‐1 vs. CHN‐1 + UFD‐2 with increasing time] (*P < 0.05; **P < 0.01; ***P < 0.001).
-
BCHN‐1 auto‐Ub was performed in the presence of UFD‐2 with the increasing molar concentration as indicated. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Right, signal quantification of the unmodified CHN‐1 (yellow), ubiquitylated fraction < 70 kDa (cyan) and > 70 kDa (magenta), plotted as a percentage of different CHN‐1 species present in the indicated condition. Plotted data are the mean from the three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (**P < 0.01).
-
CAuto‐Ub was performed as indicated using recombinant CHN‐1 and UFD‐2P951A, UBE2D1 E2, UbWT, UbNoK, or Ub with substitutions of lysines 29, 48, and 63 to arginines (Ub3KTR). Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
DSurface plasmon resonance (SPR) sensorgrams of the interaction between linear di‐Ub (M1‐ linear from UbiQ) and C. elegans UFD‐2 (magenta) or S. cerevisiae Ufd2p (cyan). Y‐axis: Response unit (RU) value. X‐axis: nmolar (nM) concentration of linear di‐Ub.
-
ECHN‐1 auto‐Ub was performed as indicated in the presence of recombinant C. elegans UFD‐2 or S. cerevisiae Ufd2p and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
Data information: Representative immunoblots for at least three independent experiments are shown in the panels.
