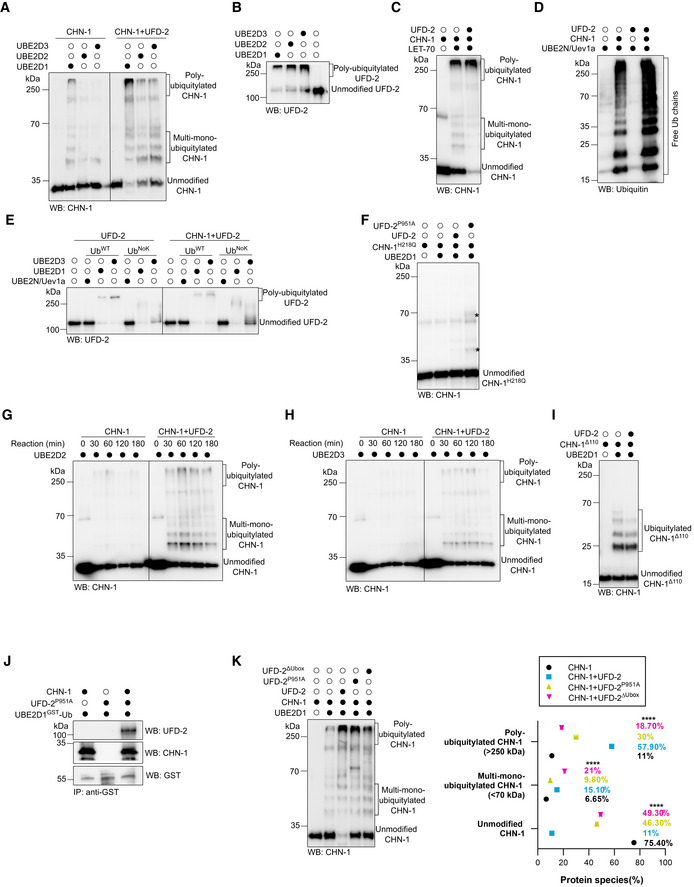
Figure EV1. Interaction with UFD‐2 increases the ubiquitylation activity of CHN‐1.
-
AAuto‐Ub of recombinant CHN‐1 only and in the presence of UFD‐2 was carried using UBE2D1, UBE2D2, or UBE2D3 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
BAuto‐Ub of recombinant UFD‐2 was performed using UBE2D1, UBE2D2, or UBE2D3 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐UFD‐2 antibodies.
-
CAuto‐Ub of recombinant CHN‐1 only and in the presence of UFD‐2 was carried using LET‐70 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
DIn vitro ubiquitylation assay performed in the presence of CHN‐1, UFD‐2 or both as indicated using UBE2N‐Uev1a E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐Ub antibodies.
-
EAuto‐Ub of recombinant UFD‐2 only and in the presence of CHN‐1 was performed as indicated using UBE2N‐Uev1a, UBE2D1, or UBE2D3 E2, and UbWT or UbNoK. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐UFD‐2 antibodies.
-
FAuto‐Ub of recombinant CHN‐1H218Q was performed in the presence of UFD‐2 or UFD‐2P951A as indicated using UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Asterisk (*) on the blot represented the signal that appeared in the presence of UFD‐2P951A.
-
GTime‐dependent (0, 30, 60, 120, and 180 min) auto‐Ub of CHN‐1 only and in the presence of UFD‐2 was performed as indicated using UBE2D2 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
HTime‐dependent (0, 30, 60, 120, and 180 min) auto‐Ub of CHN‐1 only and in the presence of UFD‐2 was performed as indicated using UBE2D3 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
IAuto‐Ub of recombinant CHN‐1Δ110 only and in the presence of UFD‐2 was performed as indicated using UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
JCo‐immunoprecipitation of ubiquitin‐charged GST‐UBE2D1 from a mixture of ubiquitin‐charged GST‐UBE2D1 and CHN‐1, ubiquitin‐charged GST‐UBE2D1 and UFD‐2P951A, or the ternary mixture of ubiquitin‐charged GST‐UBE2D1, CHN‐1 and UFD‐2P951A using Dynabeads conjugated with anti‐GST antibody. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐GST, anti‐UFD‐2, and anti‐CHN‐1 antibodies.
-
KAuto‐Ub of recombinant CHN‐1 only and in the presence of UFD‐2, UFD‐2P951A, or UFD‐2ΔUbox was performed as indicated using UBE2D1 E2. Bands were labeled as Unmodified CHN‐1, Multi‐mono‐Ubiquitylated CHN‐1, and Poly‐Ubiquitylated CHN‐1. Right, quantification of CHN‐1 modifications (Unmodified, Multi‐mono‐ubiquitylated, Poly‐ubiquitylated) plotted as percentages. Graph plotted for CHN‐1 alone (black), CHN‐1 + UFD‐2 (cyan), CHN‐1 + UFD‐2P951A (yellow), or CHN‐1 + UFD‐2ΔUbox (magenta). Plotted data are the mean of three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (****P < 0.0001).
Data information: Representative immunoblots for at least three independent experiments are shown in the panels.
