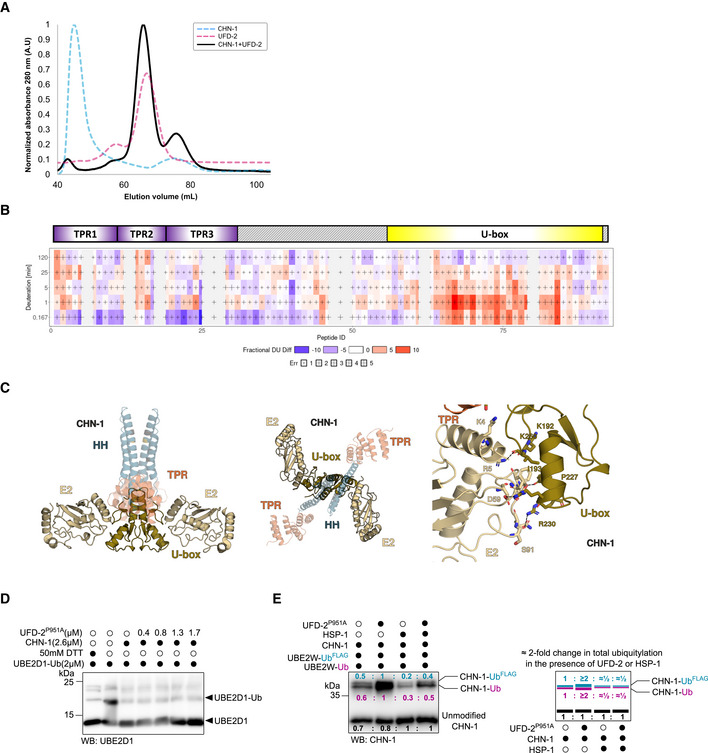
Figure EV2. UFD‐2, unlike HSP‐1, potentiates E2 accessibility of CHN‐1.
-
ASize‐exclusion chromatography (SEC) profiles of the recombinant proteins CHN‐1 (cyan), UFD‐2 (magenta), and CHN‐1 + UFD‐2 mixture (black) resolved in the S200 Superdex column.
-
BChiclet plot showing the differences in deuterium uptake by CHN‐1 peptides due to the presence of UFD‐2 across the five time points. The X‐axis spans the peptide length of CHN‐1 and the time points are plotted on the Y‐axis (total of 99 peptides with 84.2% sequence coverage and 4.55 redundancy). Above the chiclet plot is the domain organization of CHN‐1, indicating TPR and U‐box domains.
-
CModel of the CHN‐1 U‐box dimer with two E2 enzymes. UbcH5 E2 (gold) (PDB ID: 2OXQ) was aligned to the co‐crystal structure of CHIP (Danio rerio). The two structures aligned with low RMSD = 0.376. Marked are conserved residues that stabilize the critical interaction between the U‐box domain and E2.
-
DDischarging assay of Ub‐charged UBE2D1 was carried with increasing molar concentrations of recombinant UFD‐2P951A as indicated. The reaction was stopped after 30 min via the addition of Laemmli sample buffer. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐UBE2D1antibodies.
-
ECHN‐1 auto‐Ub was performed as indicated in the presence of Ube2W‐Ub or Ube2W‐UbFLAG with and without a complexing equimolar concentration of recombinant CHN‐1 and UFD‐2P951A and in the absence or presence of HSP‐1. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. For each sample, the quantified relative signal after probing the blot using anti‐CHN‐1 antibodies is represented as a ratio above the respective signal. Right, schematic of the CHN‐1‐UbFLAG (cyan), CHN‐1‐Ub (magenta), and unmodified CHN‐1 signal (black) presented as ratio and the signal fold change among CHN‐1, CHN‐1 + UFD‐2P951A, CHN‐1 + HSP‐1, and CHN‐1 + UFD‐2P951A + HSP‐1.
Data information: Representative immunoblots for at least three independent experiments are shown in the panels. Source data for HDX‐MS measurements are available in the Table EV1.
