Figure 3. UFD‐2 stabilizes an open/active CHN‐1 conformation and HSP‐1 stabilizes a closed/non‐active CHN‐1 conformation.
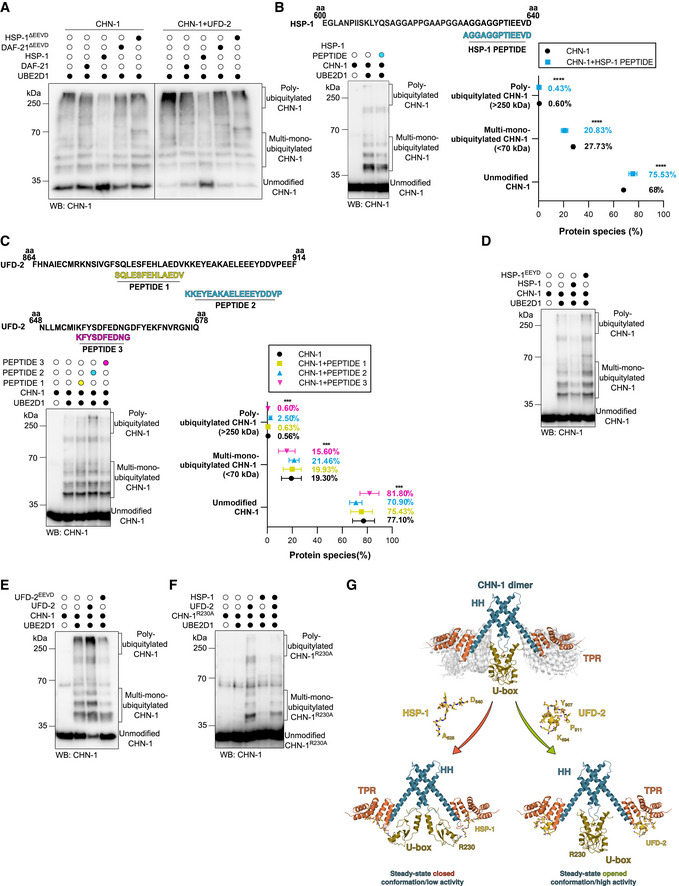
-
AAuto‐Ub of recombinant CHN‐1 using UBE2D1 E2 was performed as indicated, alone or when complexed with UFD‐2, and in the presence of recombinant DAF‐21, DAF‐21ΔEEVD, HSP‐1, or HSP‐1ΔEEVD. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
BTop, schematics of the HSP‐1 peptide sequence (cyan) aligned with the C‐terminal sequence of full‐length HSP‐1 (amino acids 600–640) used in the ubiquitylation reaction. Auto‐Ub of recombinant CHN‐1 was performed as indicated in the presence of HSP‐1‐derived peptide and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Right, quantification of the CHN‐1 modifications (Unmodified, Multi‐mono‐ubiquitylated, Poly‐ubiquitylated) when CHN‐1 alone (black) or CHN‐1 + HSP‐1 peptide (cyan). Plotted data are the mean of three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (****P < 0.0001).
-
CTop, schematics of the UFD‐2 peptide sequences aligned with the C‐terminal sequence of full‐length UFD‐2 (amino acids 864–914) for PEPTIDE 1 (yellow) and PEPTIDE 2 (cyan), and (UFD‐2 amino acids 648–678) for PEPTIDE 3 (magenta) used in the ubiquitylation reaction. Below, auto‐Ub of recombinant CHN‐1 was performed as indicated in the presence of UFD‐2‐derived peptides using UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Right, quantification of the CHN‐1 modifications (Unmodified, Multi‐mono‐ubiquitylated, Poly‐ubiquitylated) when CHN‐1 alone (black), CHN‐1 + PEPTIDE 1 (yellow), CHN‐1 + PEPTIDE 2 (cyan), or CHN‐1 + PEPTIDE 3 (magenta). Plotted data are the mean of three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (***P < 0.001).
-
DAuto‐Ub of recombinant CHN‐1 was performed as indicated using UBE2D1 E2 in the presence of recombinant HSP‐1 or HSP‐1EEYD. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
EAuto‐Ub of recombinant CHN‐1 was performed as indicated using UBE2D1 E2 in the presence of recombinant UFD‐2 or UFD‐2EEVD. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
FCHN‐1R230A auto‐Ub was performed as indicated using UBE2D1 E2 in the presence of recombinant UFD‐2 and HSP‐1. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
GModel of UFD‐2 activation and HSP‐1 inhibition of CHN‐1. Dimeric CHN‐1 with the TPR domain, U‐box, and helix‐turn‐helix (HH) is indicated by magenta, gold, and cyan, respectively. UFD‐2 and HSP‐1 peptides are shown in yellow with the indicated amino acid positions in the full‐length proteins.
Data information: Representative immunoblots for at least three independent experiments are shown in the panels.
