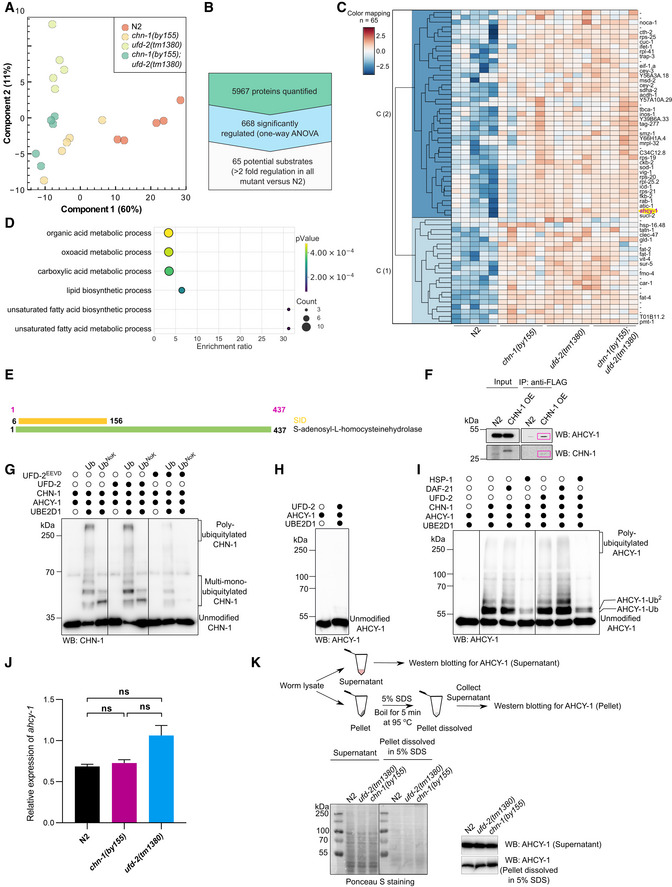
Figure EV4. Poly‐ubiquitylation of AHCY‐1 is mediated by the CHN‐1‐UFD‐2 complex.
-
APCA showing the first and second principal components of the significantly altered proteins (ANOVA FDR < 0.05) performed in the Perseus software (Tyanova et al, 2016). The percentage of explained variance is represented on the axis labels.
-
BSchematic representation of the number of identified proteins in a single‐shot analysis of LC‐MS/MS gradients in five biological replicates that led to the identification of proteins with significant abundance changes in chn‐1(by155), ufd‐2(tm1380), and chn‐1(by155); ufd‐2(tm1380) worms (twofold enrichment in all mutants versus wild‐type N2 animals).
-
CHierarchical clustering of the Z‐scores of proteins whose levels increased in chn‐1(by155), ufd‐2(tm1380), and chn‐1(by155); ufd‐2(tm1380) mutant worms (twofold enrichment in all mutants versus wild‐type N2 animals from the LC‐MS/MS experiment).
-
DGene ontology biological process terms found to be associated with C. elegans genes upregulated (minimum twofold enrichment versus N2 (control), with FDR < 0.05 for ANOVA or pairwise t‐test) in all mutants; all proteins detected in the LC‐MS/MS analysis comprised a reference set. Overrepresentation analysis was performed using the WebGestalt web server with default parameters (Liao et al, 2019). FDR was controlled to 0.25 using the Benjamini‐Hochberg method for multiple testing.
-
EYeast two‐hybrid prey fragment analysis. Schematic representations of the AHCY‐1 fragments interacting with CHN‐1 identified in the yeast two‐hybrid screen (Hybrigenics). The coding sequence for CHN‐1 was used as bait to screen a random‐primed C. elegans mixed‐stage cDNA library. The selected interaction domain (SID) is shown in yellow.
-
FCo‐immunoprecipitation of AHCY‐1 from young adult worms expressing CHN‐1::FLAG using beads conjugated with anti‐FLAG antibody. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 and anti‐FLAG antibodies (the red boxes mark the protein band).
-
GCHN‐1 auto‐Ub was performed as indicated. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
-
HUbiquitylation of recombinant AHCY‐1 was performed as indicated using recombinant UFD‐2 and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies.
-
IUbiquitylation of recombinant AHCY‐1 was performed as indicated using recombinant CHN‐1, UFD‐2, DAF‐21, or HSP‐1 in the presence of UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies.
-
JQuantitative PCR analyses of ahcy‐1 transcript levels in young adult N2 (wild‐type; black), chn‐1(by155) (magenta) and ufd‐2(tm1380) (cyan) worms. Plotted data are the mean of three biological replicates. Error bars represent SEM; statistical significance was determined using a one‐way ANOVA test.
-
KProtein level of endogenous AHCY‐1 in N2 (wild‐type), chn‐1(by155), and ufd‐2(tm1380) young adult worms. After centrifugation, the supernatant obtained from the worm lysate and the resulting pellet were dissolved in 5% SDS and boiled for 5 min. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies.
Data information: Representative immunoblots for at least three independent experiments are shown in the panels.
