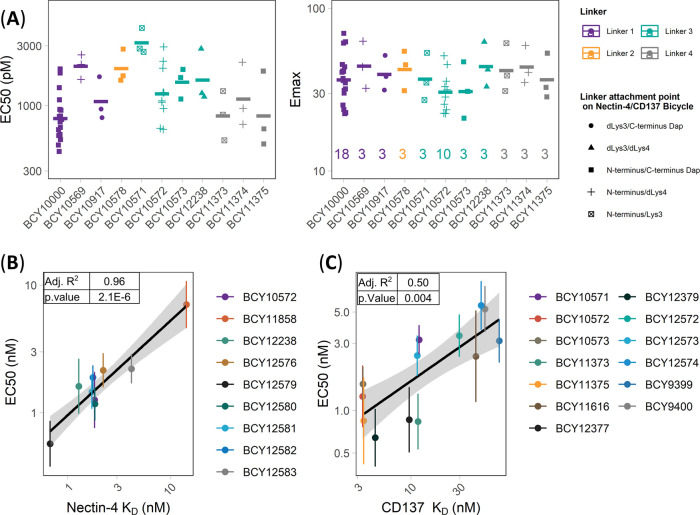
Figure 3.
Exploring the structure–activity relationship of 1:1 Nectin-4/CD137 Bicycle TICA. (A) Nectin-4/CD137 Bicycle TICAs containing the same CD137 and Nectin-4 binders, but different linker lengths and attachment point (structures in Figure 2) were assessed in the HT1376/CD137 reporter coculture assay. Individual EC50 and Emax (fold induction over background) were reported as points, geometric means as crossbars, and the number of replicates (n) shown on the Emax plot. Colors represent the linkers and shapes represent the attachment points on the Nectin-4/CD137 Bicycles (B, C) EC50 (nM) determined in HT1376/CD137 reporter coculture assay of Bicycle TICAs that have the (B) same CD137 Bicycles (BCY8928) but different Nectin-4 Bicycles or (C) same Nectin-4 Bicycle (BCY8116) but different CD137 Bicycles plotted against binding affinities (KD) to (B) Nectin-4 and (C) CD137 as measured by SPR. EC50 was reported from at least three independent experiments. SPR (KD) was measured using at least four concentrations of Bicycle TICA to obtain kon, koff, and KD. Adjusted R2 and p value are reported from the linear regression model of mean EC50 (nM) vs KD (nM).