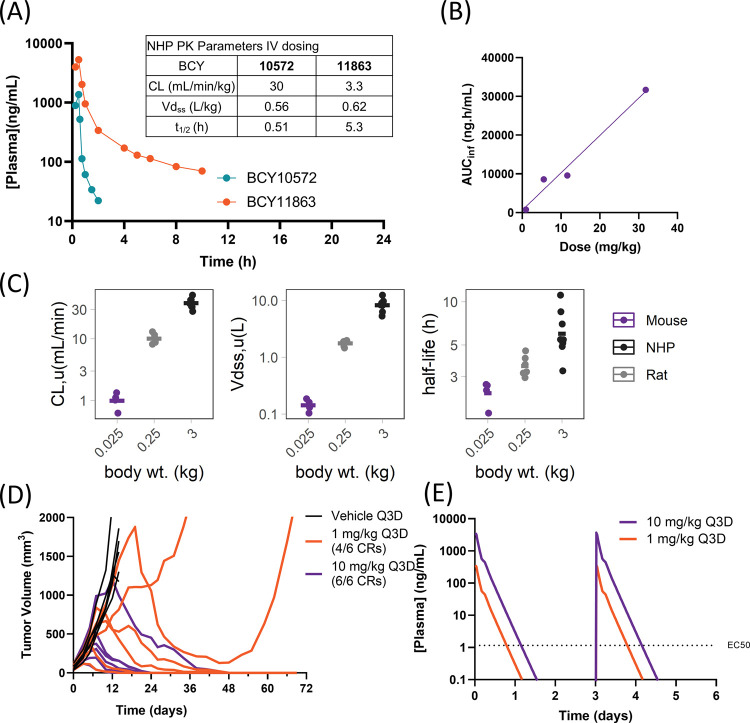
Figure 9.
Bicycle TICA pharmacokinetics across preclinical species and antitumor activity in the MC38 syngeneic model. (A) Plasma concentration–time profile and pharmacokinetic parameters (table insert) after infusion of 1 mg/kg of BCY10572 or BCY11863 over 30 min in NHPs (cynomolgus monkeys). Data are mean (n = 2 animals/compound). (B) Exposure (AUCinf) of BCY11863 plotted as a function of dose after administration of 1, 5, 10, and 30 mg/kg nominal dose of BCY11863 in CD-1 mice. (C) Unbound clearance (CL), volume of distribution (Vdss), and terminal half-life of BCY11863 plotted against mean body weight (wt) of mouse (n = 4), rat (n = 6), and NHPs (n = 8). (D) MC38-Nectin-4 tumor growth in huCD137 C57Bl/6 mice with Q3D dosing of vehicle or BCY11863 (n = 6/cohort, last dose given on day 15). The number of complete responders (CRs) is indicated in the figure. (E) Simulated plasma concentration–time profile of BCY11863 after multiple doses at 1 and 10 mg/kg Q3D in mice.