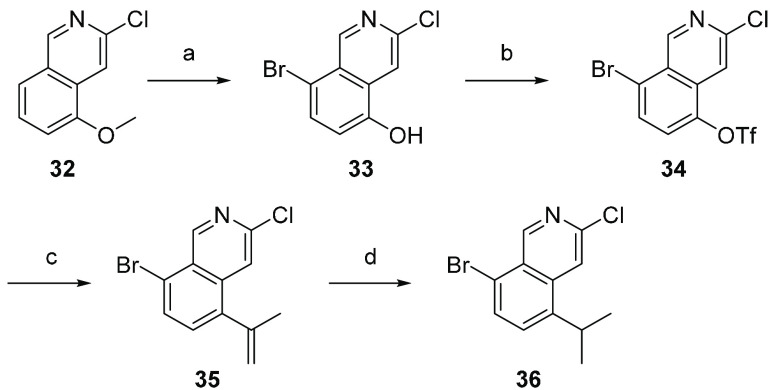
Scheme 1. Synthesis of Isoquinoline Core 36.
Reagents and conditions: (a) Br2, AcOH, rt followed by BBr3, CH2Cl2, 0 °C–rt, 75%; (b) Tf2O, TEA, CH2Cl2, −60 °C, 85%; (c) 4,4,5,5-tetramethyl-2-(prop-1-en-2-yl)-l,3,2-dioxaborolane, K2CO3, Pd(dppf)Cl2·CH2Cl2, dioxane, H2O, 45 °C, 67%; (d) PtO2, H2, EtOAc, rt, 93%.