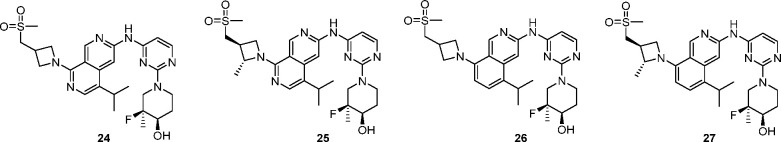
Table 3. Strategies to Improve Bioavailabilitya.
| compound |
||||
|---|---|---|---|---|
| 24 | 25 | 26 | 27 | |
| Enz EGFR LR/TM IC50 (nM) | 0.1 | 0.2 | 0.2 | 0.3 |
| Enz EGFR LR/TM/CS IC50 (nM) | 0.1 | 0.2 | 0.2 | 0.2 |
| Enz EGFR WT IC50 (nM) | 1050 | 270 | 385 | 505 |
| pEGFR H1975 LR/TM IC50(nM) | 4.8 | 1.7 | 2.7 | 1.0 |
| pEGFR A431 WT IC50 (nM) | 1608 | 781 | 1362 | 1780 |
| MDCK-MDR1 PA-B/efflux | 2/32 | 5/16 | 17/4 | 9/3 |
| rat IV PKb Cl (mL min–1 kg–1) (Clu)c, t1/2, F (%) | 67 (838), 1.6 h, 2% | – | 20 (833), 3.0 h, 85% | 25 (847), 1.3 h, 50% |
Biochemical assays using different EGFR variants measure inhibition in the presence of 1 mM ATP, and compounds were incubated with enzymes for 10 min before ATP and peptide substrate were added (for more details see Experimental Section). EGFR LR/TM means EGFR L858R/T790M, and EGFR LR/TM/CS means L858R/T790M/C790S. HLM Clint is the measurement of intrinsic clearance obtained from isolated human liver microsomes. H1975 is a gefitinib resistance human cancer cell line harboring the EGFR L858R/T790M mutation. A431 is a cell line in which EGFR is amplified.
Sprague–Dawley rats (n = 3) were dosed at 1 mg/kg IV and 5 mg/kg PO dose using the following formulations. For 24: IV, solution of 10% DMSO, 10% solutol, 80%–“20% HP-β-CD in water” PO; suspension of “20% solutol in “0.5% MC in water”. For 26 and 27: IV and PO solution of 10% DMSO, 10% solutol, 80% “20% HP-β-CD in saline”.
Clu: unbound in vivo clearance (in vivo rat clearance/free fraction in rat), free fraction calculated from plasma protein binding determined by ultracentrifugation method.