Abstract
Background
Continuous comprehensive treatment is still needed after endoscopic sinus surgery (ESS) for chronic rhinosinusitis (CRS) to promote the recovery of sinus mucosal morphology and function. Traditional Chinese medicine (TCM) nasal irrigation is a promising external treatment of TCM, but at present, the application of TCM nasal irrigation after ESS for CRS has not been recommended by the guidelines. Therefore, this article aims to develop a systematic overview and meta-analysis protocol to assess the effectiveness and safety of Chinese herbal nasal rinse for CRS recovery after ESS.
Methods
Seven databases shall be retrieved from their inception until December 2021. Eligible randomized controlled trials will be covered in the study. The outcome indicators of the survey will consist of efficacy, visual analogue scale score, Lund-Kennedy score for nasal endoscopy, Lund-Mackay score for sinus computed tomography and other secondary outcome indicators. The selection of literature, extraction of data, and methodological quality evaluation of literature shall be conducted by two researchers separately. If there is any dispute, it can be discussed and solved by a third researcher. Review Manager 5.3 software will be applied to data analysis.
Results
The article will make a detailed research programme to explore the efficacy and safety of TCM nasal irrigation on CRS recovery after ESS.
Conclusion
This protocol is suitable for evaluating the effectiveness and safety of TCM nasal rinse for CRS recovery after ESS, and can provide corresponding evidence-based medical evidence.
Systematic review registration
Open Science Framework Registration DOI: 10.17605/OSF.IO/ZV73Q.
Introduction
Chronic rhinosinusitis (CRS) is a common disease in otorhinolaryngology, impacting 5–12% of the general population [1]. The overall prevalence of CRS in the Chinese population is 8% [2,3], slightly lower than 10.9% in Europe [4] and 12% in the United States [5]. CRS is a highly heterogeneous disease, and its pathogenesis is related to the chronic obstruction of the ostiomeatal complex caused by anatomical differences, viral and bacterial infections, allergies, genetics and other factors [6]. The Chinese epidemiological survey data showed that 11.2% of CRS patients had asthma, and 27.3% had airway hyperresponsiveness [7]. A systematic review conducted by Antonino et al. showed that CRS was closely associated with atopy (49.9%), asthma (50.33%), samter triad (4.9%), and eosinophilia (4.28%) [8]. CRS greatly affects the social economy and patients’ quality of life. In the United States, the total direct costs associated with CRS range from $10 billion to $13 billion each year; the total overhead cost of job productivity losses associated with CRS is estimated to exceed $20 billion per year [9]. In addition, persistent symptoms can also affect the patient’s life and work, and even lead to problems such as depression or anxiety.
At present, there are two main treatment methods for chronic sinusitis: drug therapy and surgery. After ineffective drug treatment, endoscopic sinus surgery (ESS) is a standard surgical treatment method. The endoscopic treatment represents the best therapeutic option that the surgeon can offer for invasiveness and safety, allowing quick post-surgical, less postoperative pain and fewer complications [10]. However, surgery cannot change the inflammatory nature of the sinus mucosa, and continuous surgical cavity nursing and comprehensive drug therapy are still required to promote the gradual recovery of the morphology and function of the sinus mucosa [11]. In comprehensive postoperative treatment, seeking more effective and ideal treatment measures to stimulate the healing of the mucosal morphology and function of the surgical cavity is an important research topic faced by contemporary rhinologists.
As an essential adjuvant therapy after ESS, nasal irrigation has achieved specific curative effects in clinical application and has the advantage of fewer side effects. In addition to mechanical drainage to clean the postoperative nasal cavity, nasal irrigation can also reduce sinus mucosal inflammation, improve mucociliary clearance, and accelerate the recovery of sinus mucosal structure and function [12–15]. At present, postoperative nasal irrigation solutions include normal saline, hypertonic saline, normal saline with corticosteroids and (or) antibiotics, Chinese medicine liquid, etc. Normal saline causes no irritation to the nasal mucosa and is the most commonly used nasal rinse after surgery, but its curative effect is limited. Hypertonic saline can reduce nasal mucosal edema, but a high concentration of saline will inhibit the motor function of cilia. Jiao et al. found that hypertonic 3.0% saline significantly reduced the epithelial mucociliary or barrier function of cultured human nasal epithelial cells. Moreover, it had apparent cytotoxic effects on human nasal epithelial cells [16]. Glucocorticoids have a significant effect on reducing mucosal edema and polyp formation in the surgical cavity and promoting mucosal recovery [17]. A meta-analysis of studies by Yoon et al. showed that the beneficial effect of additional steroids in saline irrigation compared with saline irrigation alone was equivocal in terms of endoscopic score and CRS-related quality of life improvement [18]. Soudry et al. revealed that long-term nasal rinse with budesonide is generally safe, but some patients may experience asymptomatic hypothalamic-pituitary-adrenal axis (HPAA) suppression. Simultaneous use of a nasal steroid spray and a pulmonary steroid inhaler increases the risk of HPAA, especially with daily budesonide nasal irrigation [19].
Over the recent years, traditional Chinese medicine (TCM) nasal irrigation has gradually attracted the attention of researchers. More and more clinical trials have evaluated the effect of TCM nasal rinse on CRS recovery after ESS, indicating that it may be an effective and safe treatment method. Studies have revealed that [20–24] local nasal irrigation with TCM is effective in reducing nasal mucosa edema, promoting epithelialization of surgical cavity mucosa, and promoting the recovery of nasal mucociliary structure and motor function. Moreover, the local action mechanism of TCM is not a single antibacterial and anti-inflammatory, but also related to local immune regulation. Chinese medicine nasal irrigation has high safety and no obvious adverse reactions. Before and after treatment, the patient’s liver and kidney functions were normal. Only a few patients experienced nasal pain, nasal itching, and headache during nasal irrigation [24–26]. A systematic review published in 2011 showed the effectiveness and safety of TCM nasal irrigation for the postoperative recovery of chronic sinusitis after ESS [27]. However, many related clinical trials and new efficacy evaluation indicators have been added in recent years. Therefore, this paper aims to formulate a research plan to explore whether Chinese herbal nasal irrigation is effective and safe for the recovery of chronic sinusitis after ESS.
Methods
Protocol registration
The research protocol has been registered on the Open Science Framework (OSF) under the registration DOI 10.17605/OSF.IO/ZV73Q (http://osf.io/zt7h9).
According to the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocol (PRISMA-P) [28], We accomplished this research protocol. This research is a secondary acquisition and analysis of the data, and therefore no ethical approval or patient informed consent is required.
Inclusion and exclusion criteria
Types of research
All randomized controlled trials evaluating the effectiveness and safety of TCM nasal irrigation for CRS recovery after ESS shall be contained. The deadline for the included documents is December 31,2021. The postoperative nasal irrigation time of patients should be at least one month. If it is less than one month, the literature will not be included. Languages are confined to English and Chinese, with no restrictions on publication type and blindness. Duplicate publications, non-randomized controls, narrative reviews, case reports, animal tests, and other unrelated papers will be excluded.
Participants
All cases must meet the diagnostic criteria of this disease [11] and have undergone nasal endoscopic sinus surgery. After the operation, nasal irrigation was performed on the basis of standard treatment, regardless of sex, age, and race. Trials with allergic rhinitis shall be eliminated.
Interventions
The experimental group is irrigated with Chinese medicine, and the control group is irrigated with normal saline alone. The experimental group is given Chinese medicine combined with normal saline nasal rinse, while the control group is given normal saline plus antibiotics and (or) glucocorticoid nasal rinse. The experimental group is treated with Chinese medicine combined with normal saline plus antibiotics and (or) glucocorticoid nasal rinse, while the control group is treated with normal saline plus antibiotics and (or) glucocorticoid nasal irrigation.
Outcome indicators
Main outcome indicators: efficacy, including effective rate and cure rate, visual analogue scale score, Lund-Kennedy score of nose endoscope, and Lund-Mackay score of nasal sinus computed tomography.
Secondary outcome indicators: sino-nasal outcome test-20 scale, sino-nasal outcome test-22 scale, medical outcome study short form 36-items health survey, mucociliary transit time or mucociliary transport rate, cleaning time of surgical cavity, epithelization time of operative cavity, nasal immune function index determination, adverse reactions.
Retrieval strategies
These seven databases shall be retrieved from their inception to December 2021: PubMed, Excerpt Medical Database (Embase), Cochrane Central Register of Controlled Trials (CENTRAL), Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database (VIP) and Wan Fang Database. Retrieval strategies of the PubMed database are displayed in Table 1, and the search strategies of other databases are adjusted accordingly. In addition, ongoing clinical trials can be searched in the Chinese clinical trial registry. References to all relevant papers can be searched manually to facilitate access to more experimental and related research data. We can get in touch with the author by email for more details, if necessary.
Table 1. Search strategy for PubMed.
| Number | Search terms |
|---|---|
| #1 | (chronic sinusitis) OR (chronic rhinosinusitis) OR (sinusitis) OR (rhinosinusitis) OR (nasosinusitis) OR (nasal sinusitis) |
| #2 | (endoscopic sinus surgery) OR (functional endoscopic sinus surgery) OR (endoscopic surgery) OR (nasal endoscope) OR (nose endoscope) |
| #3 | (nasal irrigation) OR (nasal cavity irrigation) OR (nasal lavage) OR (nasal douche) OR (nasal rinse) OR (nasal wash) |
| #4 | (traditional Chinese medicine) OR (Chinese medicine) OR (Chinese herb) |
| #5 | #1 AND #2 AND #3 AND #4 |
| #6 | (randomized controlled trial) OR (clinical study) OR (clinical trial) OR (controlled clinical trial) |
| #7 | #5 AND #6 |
Selection of literature
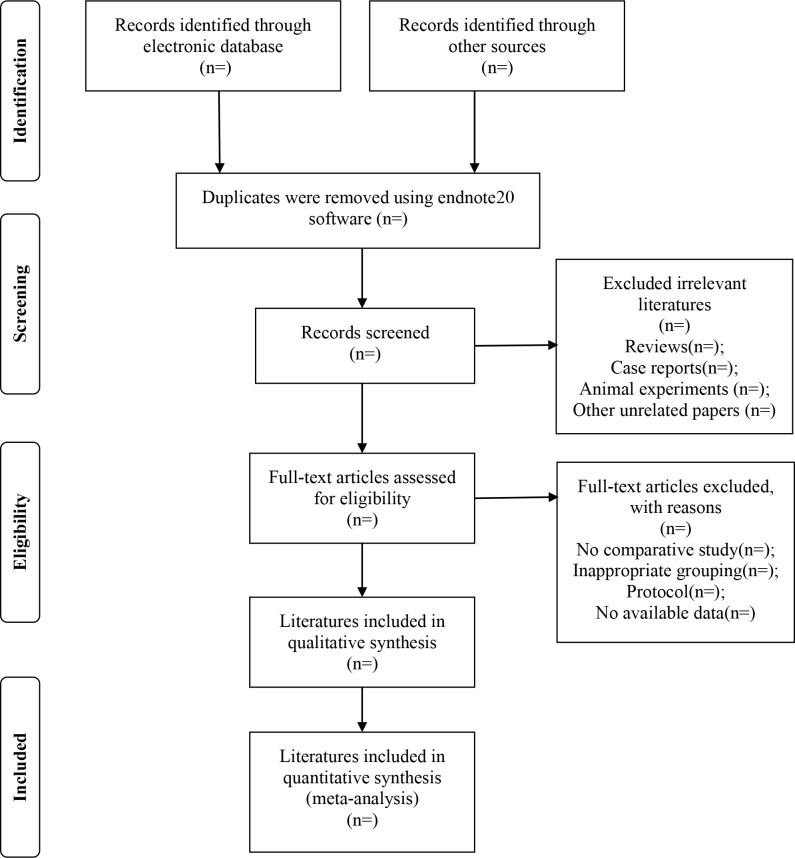
According to the above search strategy, firstly, two independent reviewers (Yang YP and Sun YN) will conduct a literature search, and obtained literature will be imported into Endnotes X9 for deduplication. Then, two independent reviewers (Yang YP and Sun YN) screen through reading headlines and summaries, download the full text of the document that initially meets the standards, and decide on the inclusion and exclusion of the document after reading the full text. If they disagree, they can discuss with the third reviewer (Zhang QX) to decide whether to include the study. The selection process of literature is based on the PRISMA flow diagram (Fig 1).
Fig 1. Flowchart of study selection.
Data extraction
Two independent investigators (Xiang F and Zhang M) will respectively extract the publication year, author’s name, sample size, patient characteristics (such as age, course of the disease, etc.), detailed information of intervention and control measures (such as the specific composition and dosage of TCM), outcome indicators and literature quality (such as randomized control method, blinding, allocation concealment, etc.), adverse reactions and other relevant information. For incomplete data, we shall contact the article’s authors to supplement associated data. For different opinions, we discuss with Zhang QX to resolve the dispute.
Methodological quality assessment
Xiang F and Fu L will evaluate the relevant literature methodology on the basis of the bias risk assessment tool in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) [29], and apply RevMan 5.3 software to produce an example graph of risk bias assessment results. If there is any disagreement, we will discuss the decision with Zhang Qinxiu.
Here are seven bias items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of subjects and researchers (implementation bias), blinding of outcome assessors (measurement bias), incomplete outcome data (follow-up bias), selective reporting (reporting bias), other bias. Each item will be judged as "low risk of bias", "high risk of bias" and "unclear" according to the risk of bias assessment criteria.
Data analysis
Review Manager 5.3 will be applied to data analysis. The risk ratio (RR) and 95% confidence interval (95%CI) will be used for the dichotomous data. For continuous measurement data, mean differences (MD) and 95% CI will be used. If the outcome measures of the study are different, the standardized mean difference (SMD) shall be adopted.
Heterogeneity will be assessed using the chi-square test and I2 statistic. If P > 0.1 or I2 < 50% indicates that there is no apparent heterogeneity, a fixed-effects model will be selected; or else, there is significant heterogeneity, and a random-effects model can be applied. For sources of heterogeneity, we can perform subgroup analyses and sensitivity analyses. According to factors such as age, course of the disease, the dose of TCM, and quality of literature, the literature can be grouped, and the literature corresponding to relevant factors can be combined for subgroup analysis to evaluate the impact on outcome indicators. Alternatively, we can eliminate the literature one by one to see if the heterogeneity has changed. If the heterogeneity changes after excluding specific literature, this literature may be the origin of heterogeneity, which may be analyzed from experimental design, sample volume, outcome indicators, etc. If the heterogeneity does not change significantly after each article is eliminated, the results are more reliable. If the data cannot be analyzed quantitatively, we shall make a qualitative description.
If more than 10 studies are included, a funnel chart will be made to determine whether there is publication bias. Finally, the strength of the evidence can be rated using the grades of Recommendations Assessment, Development and Evaluation profiler.
Discussion
CRS refers to chronic inflammation of the sinus mucosa caused by the dysfunction between all kinds of environmental aspects and the host immune system. In healthy humans, the mucosa is a barrier that modulates interactions with the host immune system, promotes tolerance and symbiosis, and prevents or limits inflammation [1]. In CRS patients, this barrier is penetrated, leading to chronic inflammation [1]. The mucociliary clearance function, humidification and filtration function of inflammatory nasal mucosa will reduce. Patients may develop inferior turbinate hypertrophy and nasal congestion and do not respond to standard medical therapy [30]. Endoscopic sinus surgery can solve the problem of sinus ostium obstruction and drainage, and create the necessary conditions for the benign outcome of sinus mucosal inflammation. However, after ESS, the operative cavity is exposed, secretions are retained, and crusts are accumulated, and some new lesions may occur, such as mucosal edema, vesicle formation, and polyp regeneration. The nasal mucosa transformation after ESS includes the following four stages: cleaning of the surgical cavity, mucosal transition, complete epithelialization, and tissue remodeling [31]. Therefore, promoting the benign outcome and epithelialization of the mucosa of the surgical cavity and reducing mucosal edema, vesicles, granulation, and other lesions during the postoperative outcome stage is an essential link to treating chronic sinusitis.
Chinese medicine nasal irrigation is a kind of external treatment of TCM, which has the unique advantages of TCM prevention and treatment. While cleaning the nasal cavity, it can also be absorbed and administered through local mucosa to achieve local and systemic therapeutic effects. Chronic sinusitis belongs to the category of "biyuan" in TCM. Chinese medicine believes that the primary pathogenesis of biyuan is wind-heat in the lung meridian, stagnant-heat in the gallbladder, and damp-heat in the spleen and stomach. Therefore, at present, TCM washing liquids are mainly selected with Chinese medicines with the functions of clearing heat and promoting diuresis, purging intense heat and detoxicating, activating circulation and removing blood stasis, and clearing the nasal cavity. Their curative effects have also been well reflected in clinical practice. Sihuang cangtao decoction is composed of coptis, cortex phellodendri, radix scutellariae, cocklebur fruit, peach seed, etc. It has the effects of clearing away heat and expelling pus, tonifying spleen and excreting dampness, activating blood to remove stasis, and dispersing and relieving nose orifice. Sihuang cangtao decoction nasal irrigation can control the inflammatory exudation and scab production of surgical wounds in patients with chronic sinusitis after the operation, prevent granulation tissue hyperplasia and vesicle formation, and improve the symptoms and signs scores of patients after the operation without noticeable adverse reactions [32]. The drug composition of Tongqiao flushing formula includes radix astragali, peach seed, flos magnoliae, radix angelicae dahuricae, platycodon grandiflorum, spina gleditsiae, flos chrysanthemi indici, etc., which has the functions of activating blood to remove stasis, draining the pus and clearing the orifices. Tongqiao irrigation formula used for nasal irrigation after ESS for chronic sinusitis can significantly shorten the epithelization time of the operation cavity, accelerate the recovery of the operation cavity mucosa and improve the postoperative symptoms and signs [33]. In addition, some modern pharmacological studies have proved that flos magnoliae, cocklebur fruit, radix angelicae dahuricae, radix scutellariae, coptis, cortex phellodendri, peach seed and flos chrysanthemi indici have anti-inflammatory effects [34–41]. Coptis polysaccharide can enhance the phagocytosis of macrophages, promote the differentiation, development and maturation of T cells, and play an immunomodulatory role [42]. Radix astragali and peach seed have a two-way regulating effect on the immune system. Aiming at the situation of low immunity, it can improve the immune function of the body. For the inflammatory response caused by hyperimmunity, it can inhibit the inflammatory response of the body [43,44].
Nasal irrigation is an effective auxiliary means for the treatment of chronic sinusitis after ESS. It is beneficial to postoperative nursing and nasal mucosal repair. In 2015, "AAO-HNS Clinical Practice Guidelines: Adult Sinusitis " pointed out that clinicians recommend nasal saline irrigation as an adjuvant treatment for chronic sinusitis, which can moisturize nasal mucosa, remove blood clots and scabs and promote mucosal healing after sinusitis surgery [45]. However, the relevant guidelines do not give opinions on TCM nasal irrigation, which may be caused by the deficiency of evidence-based medical evidence. Thus, this research aims to formulate a study protocol on the effectiveness and safety of TCM nasal irrigation on the recovery after ESS for chronic sinusitis, in order to offer more evidence-based medical support for the use of TCM nasal rinse after ESS for chronic sinusitis.
Supporting information
(DOC)
Abbreviations
- CRS
chronic rhinosinusitis
- ESS
endoscopic sinus surgery
- TCM
traditional Chinese medicine
- HPAA
hypothalamic-pituitary-adrenal axis
- PRISMA-P
Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocol
- Embase
Excerpt Medical Database
- CENTRAL
Cochrane Central Register of Controlled Trials
- CBM
Chinese Biomedical Literature Database
- CNKI
China National Knowledge Infrastructure
- VIP
Chinese Science and Technology Periodical Database
- RR
relative risk
- CI
onfidence interval
- MD
mean differences
- SMD
standardized mean difference
Data Availability
Deidentified research data will be made publicly available when the study is completed and published.
Funding Statement
This work was supported by a Grant from Xinglin Scholars Scientific Research Promotion Plan of Chengdu University of Traditional Chinese Medicine-Innovation team of traditional Chinese medicine otorhinolaryngology discipline, natural science(XKTD2021003). URL:https://www.cdutcm.edu.cn/. The funders had and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi: 10.4193/Rhin20.600 [DOI] [PubMed] [Google Scholar]
- 2.Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ, Zhu DD, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70(5):533–9. doi: 10.1111/all.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Chen J, Cheng L, Li H, Liu S, Lou H, et al. Chinese Society of Allergy and Chinese Society of Otorhinolaryngology-Head and Neck Surgery Guideline for Chronic Rhinosinusitis. Allergy Asthma Immun. 2020;12(2):176–237. doi: 10.4168/aair.2020.12.2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in European underestimated disease. A GA2LEN study. Allergy. 2011;66(9):1216–23. doi: 10.1111/j.1398-9995.2011.02646.x [DOI] [PubMed] [Google Scholar]
- 5.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014;(260):1–161. Available from: https://pubmed.ncbi.nlm.nih.gov/24819891/. [PubMed] [Google Scholar]
- 6.Heath J, Hartzell L, Putt C, Kennedy JL. Chronic Rhinosinusitis in Children: Pathophysiology, Evaluation, and Medical Management. Curr Allergy Asthma Rep. 2018;18(7):37. Epub 20180529. doi: 10.1007/s11882-018-0792-8 [DOI] [PubMed] [Google Scholar]
- 7.Katotomichelakis M, Tantilipikorn P, Holtappels G, De Ruyck N, Feng L, Van Zele T, et al. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy. 2013;27(5):354–60. doi: 10.2500/ajra.2013.27.3922 [DOI] [PubMed] [Google Scholar]
- 8.Antonino M, Nicolò M, Jerome Renee L, Federico M, Chiara V, Stefano S, et al. Single-nucleotide polymorphism in chronic rhinosinusitis: A systematic review. Clin Otolaryngol. 2022;47(1):14–23. doi: 10.1111/coa.13870 [DOI] [PubMed] [Google Scholar]
- 9.Rudmik L. Economics of Chronic Rhinosinusitis. Curr Allergy Asthm R. 2017;17(4):20. doi: 10.1007/s11882-017-0690-5 [DOI] [PubMed] [Google Scholar]
- 10.Maniaci A, Merlino F, Cocuzza S, Iannella G, Vicini C, Cammaroto G, et al. Endoscopic surgical treatment for rhinogenic contact point headache: systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2021;278(6):1743–53. doi: 10.1007/s00405-021-06724-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery. Chinese guidelines for diagnosis and treatment of chronic rhinosinusitis (2018). Chin J of Otorhinolaryngol Head Neck Surg. 2019;(02):81–100. doi: 10.3760/cma.j.issn.1673-0860.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 12.Succar EF, Turner JH, Chandra RK. Nasal saline irrigation: a clinical update. Int Forum Allergy Rhinol. 2019;9(S1): S4–s8. doi: 10.1002/alr.22330 [DOI] [PubMed] [Google Scholar]
- 13.Principi N, Esposito S. Nasal Irrigation: An Imprecisely Defined Medical Procedure. Int J Environ Res Public Health. 2017;14(5). doi: 10.3390/ijerph14050516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borish L, Baroody FM, Kim MS, Lieberman JA, Peters A, Stevens WW, et al. Yardstick for the medical management of chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2022;128(2):118–28. doi: 10.1016/j.anai.2021.10.014 [DOI] [PubMed] [Google Scholar]
- 15.Chen XZ, Feng SY, Chang LH, Lai XP, Chen XH, Li X, et al. The effects of nasal irrigation with various solutions after endoscopic sinus surgery: systematic review and meta-analysis. J Laryngol Otol. 2018;132(8):673–9. doi: 10.1017/S0022215118000919 [DOI] [PubMed] [Google Scholar]
- 16.Jiao J, Yang J, Li J, Li Y, Zhang L. Hypertonic saline and seawater solutions damage sinonasal epithelial cell air-liquid interface cultures. Int Forum Allergy Rhinol. 2020;10(1):59–68. doi: 10.1002/alr.22459 [DOI] [PubMed] [Google Scholar]
- 17.Huang ZZ, Chen XZ, Huang JC, Wang ZY, Li X, Chen XH, et al. Budesonide nasal irrigation improved Lund-Kennedy endoscopic score of chronic rhinosinusitis patients after endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2019;276(5):1397–403. doi: 10.1007/s00405-019-05327-6 [DOI] [PubMed] [Google Scholar]
- 18.Yoon HY, Lee HS, Kim IH, Hwang SH. Post-operative corticosteroid irrigation for chronic rhinosinusitis after endoscopic sinus surgery: A meta-analysis. Clin Otolaryngol. 2018;43(2):525–32. doi: 10.1111/coa.13015 [DOI] [PubMed] [Google Scholar]
- 19.Soudry E, Wang J, Vaezeafshar R, Katznelson L, Hwang PH. Safety analysis of long-term budesonide nasal irrigations in patients with chronic rhinosinusitis post endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6(6):568–72. doi: 10.1002/alr.21724 [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Wang LH, Guo Y, Zhang ZJ, Huang W, Hu R, et al. Clinical observation of nasal cavity flushing with modified buyang huanwu decoction after FESS with chronic rhinosinusitis. Lishizhen Medicine and Materia Medica Research. 2019;30(06):1420–1. doi: 10.3969/j.issn.1008-0805.2019.06.046 [DOI] [Google Scholar]
- 21.Wei C, Qin B, Wei HQ, Wei LM, Pan AN. Clinical Observation of Biyuan Lotion No.1 in Treating Biyuan. Journal of Guangxi University of Chinese Medicine. 2018;21(03):35–8. doi: 10.3969/j.issn.2095-4441.2018.03.011 [DOI] [Google Scholar]
- 22.Yang WL, Jiang YZ, Hu G, Bin J, Zhu ZH. Effect of shenling lavage fluid on morphology and function of sinusCilia in rabbits with chronic rhinosinusitis. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2017;23(02):25–7+32. doi: 10.13862/j.cnki.cn43-1446/r.2017.02.006 [DOI] [Google Scholar]
- 23.Liu FX, Ye XH, Wang LL, lin J. Effect of sinusitis mixture on mucosa cells after functional endoscopic sinus surgery. Chinese Journal of Integrated Traditional and Western Medicine. 2016;36(04):430–3. doi: 10.7661/CJIM.2016.04.0430 [DOI] [PubMed] [Google Scholar]
- 24.Ma F, Xu L, Ai P. Treatment and Impacts of Chronic Sinusitis with the Confluence of Biyuan Tongqiao Granules and Saline Nasal Irrigation. J Healthc Eng. 2022;2022:2916700. doi: 10.1155/2022/2916700 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wu H, Tang YY. Clinical observation on therapeutic effect of combined routine medical therapy with nasal douche by Biyuan Decoction on children chronic rhinosinusitis in the pattern of Lung-Spleen Qi deficiency. Chin J Otorhinolaryngol Integ Med. 2012;20(01):31–34. doi: 10.3969/j.issn.1007-4856.2012.01.009 [DOI] [Google Scholar]
- 26.Ran TT. The clinical research of Cang er zi powder modified by nasal irrigation for chronic rhinosinusitis treated after functional endoscopic sinus surgery. Fujian University of TCM. 2015.Available from: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201601&filename=1015323062.nh&uniplatform=NZKPT&v=e3l6iI_rvtXFjTNoLyZ36Xn0LGm2OO-4CcBO2mJuLAfSMDXVt8No6axO28f_YEQn. [Google Scholar]
- 27.Zhang DZ, Liu M, Zhang QX, Li XQ, Xian KL, Li JL, et al. Traditional Chinese medicine in the irrigation of chronic rhinosinusitis after endoscopic sinus surgery: a systematic review. Chin J Evid-based Med. 2011;11(05):576–90. doi: 10.3969/j.issn.1672-2531.2011.05.020 [DOI] [Google Scholar]
- 28.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical research ed). 2015;350: g7647. doi: 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 29.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. Journal of evidence-based medicine. 2015;8(1):2–10. doi: 10.1111/jebm.12141 [DOI] [PubMed] [Google Scholar]
- 30.Maniaci A, Lechien JR, La Mantia I, Calvo-Henriquez C, Iannella G, Locatello LG, et al. Effectiveness of submucosal turbinoplasty in refractory obstructive rhinitis: a prospective comparative trial. Eur Arch Otorhinolaryngol. doi: 10.1007/s00405-022-07267-0 [DOI] [PubMed] [Google Scholar]
- 31.Selvarajah J, Saim AB, Bt Hj Idrus R, Lokanathan Y. Current and Alternative Therapies for Nasal Mucosa Injury: A Review. Int J Mol Sci. 2020;21(2). doi: 10.3390/ijms21020480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang L, Li F, Tao CH, Fang HY, Chen QK, Han XZ. Effect evaluation of Sihuang Cangtao Decoction in nasal irrigation after chronic sinusitis nasal endoscopic operation. Chongqing Medicine. 2021;50(13):2233–6+41. doi: 10.3969/j.issn.1671-8348.2021.13.015 [DOI] [Google Scholar]
- 33.Zhang J, Wang LH, Sha ZR, Guo Y, Mao JJ, Wang M, et al. Observation on the curative effect of Tongqiao flushing formula on the recovery of chronic rhinosinusitis after FESS operation. Chin J Otorhinolaryngol Integ Med. 2018;26(05):372–5. doi: 10.16542/j.cnki.issn.1007-4856.2018.05.014 [DOI] [Google Scholar]
- 34.Zeng WX, Liu SJ, Wang H, Chen SZ. Assessment of anti-Inflammatory effect of standard magnolia biondii oil. Chin Pharm J. 2013;48(05):349–54. doi: 10.11669/cpj.2013.05.007 [DOI] [Google Scholar]
- 35.Liu CM, Chen HP, Tan LP, Yang K, Zeng CH. Pharmacological effect and toxicity of xanthii fructus. Chinese Journal of Experimental Traditional Medical Formulae. 2019;25(09):207–13. doi: 10.13422/j.cnki.syfjx.20190928 [DOI] [Google Scholar]
- 36.Lei YT, Huang T, Chen WL, Li HJ, Jiang GH, Deng W. Comparative Study on the anti-Inflammatory, analgesic effects and components contents of different grades of angelica dahurica var. formosana. Pharmacology and Clinics of Chinese Materia Medica. 2021;37(01):105–10. doi: 10.13412/j.cnki.zyyl.20210319.001 [DOI] [Google Scholar]
- 37.Gao GW, Li L. Study on the anti-inflammatory effect and mechanism of the extract of scutellaria baicalensis georgi. Chin J Clin Pharmacol. 2014;30(06):550–2. doi: 10.13699/j.cnki.1001-6821.2014.06.025 [DOI] [Google Scholar]
- 38.Park SM, Min BG, Jung JY, Jegal KH, Lee CW, Kim KY, et al. Combination of Pelargonium sidoides and Coptis chinensis root inhibits nuclear factor kappa B-mediated inflammatory response in vitro and in vivo. BMC Complement Altern Med. 2018;18(1):20. doi: 10.1186/s12906-018-2088-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L, Zhang YY, Li H, Wan XZ, Wu JJ. Effects of cortex phellodendri Chinensis decoction on anti-inflammatory and anti-bacteria. Laboratory Animal Science and Administration. 2014;31(04):14–7. doi: 10.3969/j.issn.1006-6179.2014.04.004 [DOI] [Google Scholar]
- 40.Chen XY. Studies on the chemical constituents and bioactivities od the seeds of prunus davidiana and the leaves of morus alba. Peking Union Medical College. 2014. Available from:https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CDFD&dbname=CDFDLAST2015&filename=1014345696.nh&uniplatform=NZKPT&v=9y8VETQ55rs8SrD6Bx1mDbv1VpOvV_IHviI2F6h9U_mgzeAq0UY-MWwdGpYDfvJr. [Google Scholar]
- 41.Jiang ZK, Li X, Luo B. Anti-inflammatory and Analgesic effects of essential oil from chrysanthemi Indici flos. Chinese Journal of Experimental Traditional Medical Formulae. 2015;21(16):124–7. doi: 10.13422/j.cnki.syfjx.2015160124 [DOI] [Google Scholar]
- 42.Ji WY, Feng XC, Qiu F, Li W. Research progress on pharmacological effects of Coptis Chinensis polysaccharide. Drug Evaluation Research. 2021;44(03):638–43. doi: 10.7501/j.issn.1674-6376.2021.03.027 [DOI] [Google Scholar]
- 43.Xu XH, Li T, Wang YT, Lu JJ. Research progress in Persicae Semen. Chinese Traditional and Herbal Drugs. 2015;46(17):2649–55. doi: 10.7501/j.issn.0253-2670.2015.17.023 [DOI] [Google Scholar]
- 44.Wang CN, Cheng DY, Wang J, Wang LS. Research progress on bidirectional immunoregulation of huangqi (astragali radix) and its compound preparations. Chinese Archives of Traditional Chinese Medicine. 2021;39(05):126–9. doi: 10.13193/j.issn.1673-7717.2021.05.031 [DOI] [Google Scholar]
- 45.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl): S1–s39. doi: 10.1177/0194599815572097 [DOI] [PubMed] [Google Scholar]