Table 1.
Reaction optimizationa
| Entry | R*SH | Solvent | Yield /%b | D /%c | erd |
|---|---|---|---|---|---|
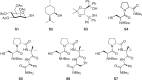
| 1 | S1 | toluene:D2O (3:1) | 56 | 96 | 48:52 |
| 2 | S2 | toluene:D2O (3:1) | 79 | 90 | 51:49 |
| 3 | S3 | toluene:D2O (3:1) | 42 | 90 | 53:47 |
| 4 | S4 | toluene:D2O (3:1) | 50 | 95 | 58:42 |
| 5 | S5 | toluene:D2O (3:1) | 67 | 94 | 93:7 |
| 6 | S6 | toluene:D2O (3:1) | 49 | 96 | 93:7 |
| 7 | S7 | toluene:D2O (3:1) | 68 | 94 | 92:8 |
| 8 | S5 | toluene:D2O (1:1) | 43 | 97 | 93:7 |
| 9 | S5 | toluene:D2O (4:1) | 71 | 90 | 93:7 |
| 10 | S5 | toluene | 73 | – | 88:12 |
| 11e | S5 | toluene:D2O (3:1) | 73 | 94 | 93:7 |
| 12f | S5 | toluene:D2O (3:1) | N.D. | – | – |
| 13g | S5 | toluene:D2O (3:1) | N.D. | – | – |
| 14h | – | toluene:D2O (3:1) | N.D. | – | – |
 | |||||
er enantiomeric ratio, N.D. Not detected.
aUnless otherwise noted, all reactions were carried with 1a (0.2 mmol), 2a (0.1 mmol), 4DPAIPN (1 mol%), R*SH (15 mol%), toluene (0.75 mL), D2O (0.25 mL) under 10 °C for 48 h with irradiation from a 30 W blue LED.
bIsolated yield of 3a.
cDetermined by 1H NMR analysis of the isolated product.
dDetermined by chiral HPLC analysis.
eReaction time: 72 h.
fNo photocatalyst.
gWithout light irradiation.
hNo thiol catalyst.
