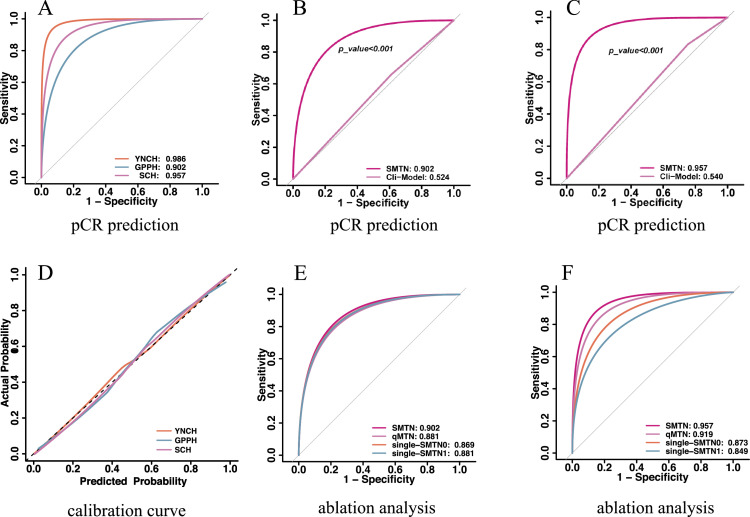
Figure 3.
Performances for pCR prediction. A: AUCs of the SMTN in the training cohort and two validation cohorts; B: AUCs of the SMTN and clinical model in the external validation cohort 1; C: AUCs of the SMTN and clinical model in the external validation cohort 2; D: The calibration curve of the SMTN in the training cohort and two validation cohorts; E: AUC of the SMTN, qMTN, single-MTN0, and single-MTN1 in the external validation cohort 1; F: AUC of the SMTN, qMTN, single-MTN0, and single-MTN1 in the external validation cohort 2. pCR: pathological complete response; ROC: receiver operating characteristics; AUC: area under the receiver operating characteristics curve; YNCH: Yunnan Cancer Hospital (training cohort); GPPH: Guangdong Provincial People's Hospital (external validation cohort 1); SCH: Shanxi Cancer Hospital (external validation cohort 2); SMTN: Siamese multi-task network; Clin-model: clinical model; single-MTN0: a multi-task network trained with ultrasound image before neoadjuvant chemotherapy; single-MTN1: a multi-task network trained with ultrasound image after the first/second cycle of neoadjuvant chemotherapy, qMTN: the dynamic information changes capture branch of SMTN was removed, and the remaining structure was used to predict pCR based on longitudinal ultrasound images.