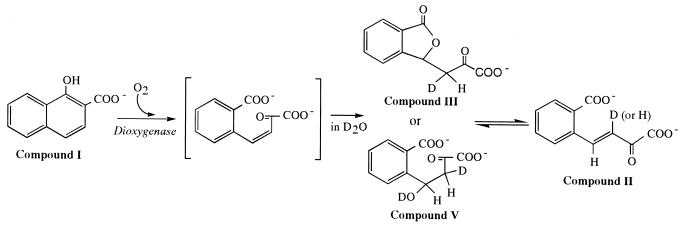
FIG. 7.
Possible mechanisms of spontaneous isomerization of a hypothetical primary product [(Z)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate] to (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate (compound II) in D2O. Compounds III and V are hypothetical intermediates in the nonenzymatic conversion from (Z)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate to (E)-4-(2-carboxylatophenyl)-2-oxo-3-butenoate.